CHEMICAL AND PHYSICAL PROPERTIES
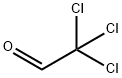
| Physical Description | Trichloroacetaldehyde appears as a colorless oily liquid with a penetrating odor. Reacts with water and denser than water. Contact may irritate skin, eyes and mucous membranes. Toxic by ingestion and inhalation. Used to make pesticides. |
|---|---|
| Color/Form | Colorless, mobile, oily liquid |
| Odor | Pungent, irritating odor |
| Boiling Point | 208 °F at 760 mmHg (NTP, 1992) |
| Melting Point | -71.5 °F (NTP, 1992) |
| Flash Point | 167 °F (NTP, 1992) |
| Solubility | Reaction (NTP, 1992) |
| Density | 1.51 at 68 °F (NTP, 1992) - Denser than water; will sink |
| Vapor Density | 5.1 (Air = 1) |
| Vapor Pressure | 35 mmHg at 68 °F ; 40 mmHg at 68.4 °F; 760 mmHg at 207.9 °F (NTP, 1992) |
| Henry's Law Constant | Henry's Law constant = 2.91X10-9 atm-cu m/mol at 25 °C |
| Stability/Shelf Life | UNSTABLE |
| Heat of Vaporization | 97.1 BTU/Lb |
| Polymerization | POLYMERIZES UNDER INFLUENCE OF LIGHT & IN PRESENCE OF SULFURIC ACID FORMING WHITE SOLID TRIMER CALLED METACHLORAL. |
| Refractive Index | Index of refraction = 1.45572 at 20 °C/D; 1.4512 at 21.4 °C/He |
| Dissociation Constants | pKa = 9.66 |
| Kovats Retention Index | 715 695 699.1 |
| Other Experimental Properties | Conversion factor: mg/cu m = 6.03 X ppm |
| Chemical Classes | Other Classes -> Aldehydes |
COMPUTED DESCRIPTORS
| Molecular Weight | 147.38 g/mol |
|---|---|
| XLogP3 | 1.6 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 0 |
| Exact Mass | 145.909298 g/mol |
| Monoisotopic Mass | 145.909298 g/mol |
| Topological Polar Surface Area | 17.1 Ų |
| Heavy Atom Count | 6 |
| Formal Charge | 0 |
| Complexity | 54.3 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Trichloroacetaldehyde appears as a colorless oily liquid with a penetrating odor. Reacts with water and denser than water. Contact may irritate skin, eyes and mucous membranes. Toxic by ingestion and inhalation. Used to make pesticides.