Celastrol
Synonym(s):10-Hydroxy-2,4a,6a,9,12b,14a-hexamethyl-11-oxo-1,2,3,4,4a,5,6,6a,11,12b,13,14,14a,14b-tetradecahydro-picene-2-carboxylic acid;Celastrol, Celastrus scandens - CAS 34157-83-0 - Calbiochem;Tripterin;Tripterin, 3-Hydroxy-24-nor-2-oxo-1(10),3,5,7-friedelatetraen-29-oic Acid, Proteasome Inhibitor XIX
- CAS NO.:34157-83-0
- Empirical Formula: C29H38O4
- Molecular Weight: 450.61
- MDL number: MFCD03424073
- EINECS: 636-472-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 13:58:55
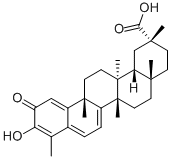
What is Celastrol?
Description
Celastrol (34157-83-0) displays potent antioxidant and anti-inflammatory activity. Inhibits NFkB (IC50=270 nM). ?It induces heat shock response and cytoprotection in various cells. Inhibits 20S proteasome chymotrypsin activity (IC50=2.5 mM). Induces autophagy by targeting AR/miR-101 in prostate cancer cells.4 Induces UPR-dependent cell death in cancer cells.5 Cell permeable.
Chemical properties
Orange Red Solid. Celastrol was initially isolated from Tripterygium wilfordii (thunder of god vine) and later identified in a variety of plant species in the Celastracae family. The natural product has a wide array of promising activities relevant to neuronal degeneration, inflammation, diseases caused by protein misfolding, cancer, and obesity.
The Uses of Celastrol
Celastrol, Celastrus scandens is an antiproliferative, antipreroxidative, and inhibits Topo II. Celastrol is an antioxidant natural product which inhibits the growth of human glioma xenografts in nude mice through suppressing VEGFR expression. Used for clinical treatment for rheumatoid arthritis, was demonstrated to have antiangiogenic activity, and be inhibitory against mice tumor growth by a few recent studies. Celastrol also has been shown to have the power to kill tumor cells and can work as an anticarcinogen. It is also a leptin sensitizer with the potential to reduce weight in obesity.
Definition
ChEBI: Celastrol is a pentacyclic triterpenoid that is 24,25,26-trinoroleana-1(10),3,5,7-tetraen-29-oic acid bearing an oxo substituent at position 2, a hydroxy substituent at position 3 and two methyl groups at positions 9 and 13. An antioxidant and anti-inflammatory agent. Potently inhibits lipid peroxidation in mitochondria and inhibits TNF-alpha-induced NFkappaB activation. Also shown to inhibit topoisomerase II activity in vitro (IC50 = 7.41 muM). It has a role as an antioxidant, an anti-inflammatory drug, an EC 5.99.1.3 [DNA topoisomerase (ATP-hydrolysing)] inhibitor, an antineoplastic agent, a Hsp90 inhibitor and a metabolite. It is a pentacyclic triterpenoid and a monocarboxylic acid.
Biological Activity
Antioxidant and anti-inflammatory agent. Potently inhibits lipid peroxidation in mitochondria and inhibits TNF- α -induced NF κ B activation. Also shown to inhibit topoisomerase II activity in vitro (IC 50 = 7.41 μ M).
Biochem/physiol Actions
Celastrol is a potent antioxidant, and anti-inflammatory agent. It is a novel HSP90 inhibitor (disrupts Hsp90/Cdc37 complex), that exhibits anticancer (anti-angiogenic - suppresses VEGFR expression); antioxidant (inhibits lipid peroxidation) and anti-inflammatory activity (suppresses iNOS and inflammatory cytokine production).
Storage
+4°C
References
1) Sethi et al. (2007), Celastrol, a novel triterpene, potentiates TNF-induced apoptosis and suppresses invasion of tumor cells by inhibiting NF-κC-regulated gene products and TAK1-mediated NF-κB activation; Blood, 109 2727
2) Westerheide et al. (2004), Celastrols as inducers of the heat shock response and cytoprotection; J. Biol. Chem., 279 56053
3) Yang et al. (2006), Celastrol, a triterpene extracted from the Chinese “Thunder of God Vine” is a potent proteasome inhibitor and suppresses human prostate cancer cell growth in nude mice; Cancer Res., 66 4758
4) Guo et al. (2015), Celastrol Induces Autophagy by targeting AR/miR-101 in Prostate Cancer Cells; PLoS One, 10(10) e0140745
5) Fribley et al. (2015), Celastrol induces unfolded protein response-dependent cell death in head and neck cancer; Exp. Cell Res., 330 412
Properties of Celastrol
| Melting point: | 219-230°C |
| Boiling point: | 645.7±55.0 °C(Predicted) |
| Density | 1.2 |
| storage temp. | -20°C |
| solubility | DMSO: >10mg/mL |
| form | solid |
| pka | 4.78±0.70(Predicted) |
| color | red |
| λmax | 424nm(MeOH)(lit.) |
| Stability: | Stable for 2 years from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20° for up to 1 month. |
| CAS DataBase Reference | 34157-83-0(CAS DataBase Reference) |
Safety information for Celastrol
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral |
| Precautionary Statement Codes |
P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for Celastrol
| InChIKey | KQJSQWZMSAGSHN-JJWQIEBTSA-N |
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

You may like
-
 Celastrol 98% (HPLC) CAS 34157-83-0View Details
Celastrol 98% (HPLC) CAS 34157-83-0View Details
34157-83-0 -
 Celastrol CAS 34157-83-0View Details
Celastrol CAS 34157-83-0View Details
34157-83-0 -
 Celastrol, Celastrus scandens CAS 34157-83-0View Details
Celastrol, Celastrus scandens CAS 34157-83-0View Details
34157-83-0 -
 Celastrol CAS 34157-83-0View Details
Celastrol CAS 34157-83-0View Details
34157-83-0 -
 Celastrol CAS 34157-83-0View Details
Celastrol CAS 34157-83-0View Details
34157-83-0 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
