Ellagic acid
Synonym(s):4,4?,5,5?,6,6?-Hexahydroxydiphenic Acid 2,6,2?,6?-Dilactone, TBBD, PRMT Inhibitor II;4,4′,5,5′,6,6′-Hexahydroxydiphenic acid 2,6,2′,6′-dilactone;Alizarin Yellow;Benzoaric acid;Elagostasine
- CAS NO.:476-66-4
- Empirical Formula: C14H6O8
- Molecular Weight: 302.19
- MDL number: MFCD00006914
- EINECS: 207-508-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 18:10:20
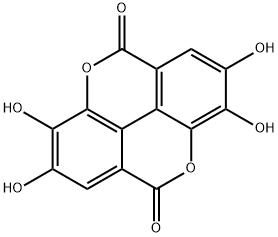
What is Ellagic acid?
Description
Ellagic acid is a natural fused-ring polyphenol with a pKa of 5.5. It is found in fruits, nuts, the galls of oak trees, and the kino (gum) of eucalyptus trees. Its name comes from the French acide ellagique, coined from the word galle (gall) spelled backward.
Ellagic acid has been known since the first half of the 19th century as a component of tanning materials. It was mentioned in the chemical literature as early as 1868, when German chemist and industrialist Julius L?we synthesized it by oxidizing gallic acid1 with arsenic acid or silver oxide. In 1905, Arthur George Perkin2* and Maximilian Nierenstein at the University of Leeds (UK) refined the process by using sodium persulfate as the oxidant.
Ellagic acid has been touted as a natural antioxidant and marketed as a dietary supplement with claims that it can prevent or treat cancer and heart disease. From 2008 to 2021, the US Food and Drug Administration issued warnings to drug companies and the public about false health claims about the molecule.
But ellagic acid may have some health benefits. In 2006, Juan Carlos Espin and colleagues at CEBAS?CSIC3 (Murcia, Spain) found that two colonic microflora ellagic acid metabolites (urolithins A4 and B5) have, oddly, both estrogenic and antiestrogenic activity. The authors suggested that the urolithins may have implications for treating breast cancer. Espin and his group subsequently investigated several aspects of urolithin activity, including its anti-inflammatory properties.
Fast forward to September 2022: Building on Espin’s work, Jian Zhao and co-workers at Sichuan University (Chengdu, China), used mouse studies to determine that urolithin A attenuates Helicobacter pylori-induced damage in vivo. Urolithin A not only decreased inflammation in the gut, but it also reduced H. pylori virulence factor secretion, tissue injuries, and the relative abundance of Helicobacter spp. In feces of H. pylori-infected mice.
For other findings on ellagic acid, see ScienceDirect’s information page.
1. CAS Reg. No. 149-91-7.
2. For whom the Perkin Transactions journals were named.
3. Center for Edaphology and Applied Biology of Segura, Spanish National Research Council. Edaphology is a type of soil science.
4. CAS Reg. No. 1143-70-0.
5. CAS Reg. No. 1139-83-9.
Chemical properties
cream to light yellow crystalline solid, soluble in methanol, ethanol, DMSO and other organic solvents, derived from Mangiferaindica, Eucalyptuscitriodora, Lythrumsalicaria, Geranium sibiricum, Agrimoniapilosavar.japonica.
The Uses of Ellagic acid
Ellagic Acid is a phenol antioxidant found naturally in various fruits and vegetables. Ellagic Acid was shown to exhibit high levels of hemostatic, antineoplastic, antimutagenic, antiproliferative and antioxidant properties in studies, which suggests its potential health benefits following ellagic acid consumption.
What are the applications of Application
Ellagic acid is used in medicine and cosmetics, as an antioxidant, and has anti-cancer and anti-viral effects.
Ellagic acid, a common plant polyphenol, is an inhibitor of glutathione S-transferase with Exhibits antitumor activity. It can be used for the determination of factor XIIa in plasma. Contact activation can occur in blood coagulation. It is also used as selective, ATP-competitive inhibitor of casein kinase 2 and a Topo I and II, FGR, GSK, and PKA inhibitor.
What are the applications of Application
Ellagic Acid, Dihydrate is a CK2, Topo I and II, FGR, GSK, and PKA inhibitor
Definition
ChEBI: Ellagic acid is an organic heterotetracyclic compound resulting from the formal dimerisation of gallic acid by oxidative aromatic coupling with intramolecular lactonisation of both carboxylic acid groups of the resulting biaryl. It is found in many fruits and vegetables, including raspberries, strawberries, cranberries, and pomegranates. It has a role as an antioxidant, a food additive, a plant metabolite, an EC 5.99.1.2 (DNA topoisomerase) inhibitor, an EC 5.99.1.3 [DNA topoisomerase (ATP-hydrolysing)] inhibitor, an EC 1.14.18.1 (tyrosinase) inhibitor, an EC 2.3.1.5 (arylamine N-acetyltransferase) inhibitor, an EC 2.4.1.1 (glycogen phosphorylase) inhibitor, an EC 2.5.1.18 (glutathione transferase) inhibitor, an EC 2.7.1.127 (inositol-trisphosphate 3-kinase) inhibitor, an EC 2.7.1.151 (inositol-polyphosphate multikinase) inhibitor, an EC 2.7.4.6 (nucleoside-diphosphate kinase) inhibitor, a skin lightening agent, a fungal metabolite, an EC 2.7.7.7 (DNA-directed DNA polymerase) inhibitor and a geroprotector. It is an organic heterotetracyclic compound, a cyclic ketone, a lactone, a member of catechols and a polyphenol. It is functionally related to a gallic acid.
Preparation
Ellagic acid is mainly extracted from plants, and it is present in several fruits such as cranberries, strawberries, raspberries, and pomegranates. Usually, the raw materials are degreasing and then extracted with an alkaline aqueous solution or extracted with ethanol. Then, after removing water-soluble proteins and gliadins, the sugar ligands can be decomposed by enzymes to obtain purer ellagic acid.
General Description
Cream-colored needles (from pyridine) or yellow powder. Odorless.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Ellagic acid reacts as a weak acid. Incompatible with strong reducing substances such as hydrides, nitrides, alkali metals, and sulfides. Flammable gas (H2) is often generated. Heat is also generated by the acid-base reaction between phenols and bases. May be sulfonated exothermically very readily (for example, by concentrated sulfuric acid at room temperature). May be nitrated very rapidly, even by dilute nitric acid.
Fire Hazard
Flash point data for Ellagic acid are not available; however, Ellagic acid is probably combustible.
Biological Activity
Selective, ATP-competitive inhibitor of casein kinase 2 (CK2) (IC 50 values are 40, 2900, 3500, 4300 and 9400 nM for CK2, Lyn, PKA, Syk? and FGR respectively). Exhibits antioxidant, antitumor and anticarcinogenic activity and also inhibits glutathione S-transferase.
Biochem/physiol Actions
Commonly occurring plant polyphenol, inhibitor of glutathione S-transferase. Used for the assay of factor XIIa in plasma. Contact activation in blood coagulation.
Anticancer Research
Ellagic acid is a naturally occurring phenolic constituent present in natural productsand nuts, most elevated amounts of which are found in raspberries (Daniel et al.1990). EA is considered as a potent anticarcinogenic and antimutagenic compound.EA shows anti-angiogenic property by repressing PDGF-R movement and phosphorylationof its substrate. It can intrude with endothelial cell-associated VEGR-2phosphorylation bringing about the restraint of the downstream signaling activatedby this receptor and in the hindrance of two key events fundamental in angiogenesis,i.e., EC movement and morphogenic separation into capillary-like structure. In parallel,EA indicated robust inhibitory activity against perivascular cells through itsrestraint of PDGF-R action and signaling prompting hindrance of VSMC relocation(Labrecque et al. 2005).
It is a phenolic compound extracted from pomegranate. It is an antiproliferative andantioxidant compound (Murakami et al. 1996). It induces apoptosis in cancer cellsof the prostate and breast and prevents the process of metastasis in different cancers(Singh et al. 2016b).
Storage
Room temperature
Purification Methods
This antioxidant crystallises from pyridine. It forms a dark green solution in aqueous N NaOH. The tetraactetate dilactone crystallises from Ac2O, with m 340o. [Beilstein 19 H 261, 19 III/IV 3164, 19/7 V 108.]
Properties of Ellagic acid
| Melting point: | ≥350 °C |
| Boiling point: | 363.24°C (rough estimate) |
| Density | 1.667 |
| refractive index | 1.5800 (estimate) |
| storage temp. | 2-8°C |
| solubility | 1 M NaOH: 10 mg/mL, dark green |
| form | powder |
| appearance | cream-colored to yellow crystals or powder |
| pka | 5.02±0.20(Predicted) |
| color | tan to gray |
| Water Solubility | <0.1 g/100 mL at 21 ºC |
| Sensitive | Air & Light Sensitive |
| Merck | 14,3547 |
| BRN | 47549 |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 476-66-4(CAS DataBase Reference) |
| EPA Substance Registry System | Ellagic acid (476-66-4) |
Safety information for Ellagic acid
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P501:Dispose of contents/container to..… |
Computed Descriptors for Ellagic acid
| InChIKey | AFSDNFLWKVMVRB-UHFFFAOYSA-N |
Ellagic acid manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran




You may like
-
 Ellagic acid 98%View Details
Ellagic acid 98%View Details -
 Ellagic acid, 95%+ CAS 476-66-4View Details
Ellagic acid, 95%+ CAS 476-66-4View Details
476-66-4 -
 Ellagic acid, technical grade CAS 476-66-4View Details
Ellagic acid, technical grade CAS 476-66-4View Details
476-66-4 -
 Ellagic acid dihydrate 99% (HPLC) CAS 476-66-4View Details
Ellagic acid dihydrate 99% (HPLC) CAS 476-66-4View Details
476-66-4 -
 Ellagic acid dihydrate 95.00% CAS 476-66-4View Details
Ellagic acid dihydrate 95.00% CAS 476-66-4View Details
476-66-4 -
 Ellagic acid CAS 476-66-4View Details
Ellagic acid CAS 476-66-4View Details
476-66-4 -
 Ellagic acid CAS 476-66-4View Details
Ellagic acid CAS 476-66-4View Details
476-66-4 -
 Ellagic Acid, Dihydrate CAS 476-66-4View Details
Ellagic Acid, Dihydrate CAS 476-66-4View Details
476-66-4
