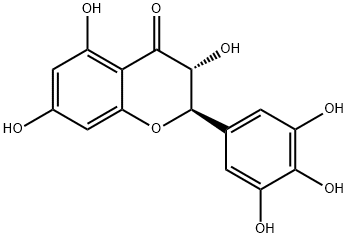
Myricetin
Synonym(s):3,3ʹ,4ʹ,5,5ʹ,7-Hexahydroxyflavone, Hsp70 Inhibitor II;3,3′,4′,5,5′,7-Hexahydroxyflavone;Cannabiscetin;Myricetin - CAS 529-44-2 - Calbiochem;Myricetol
- CAS NO.:529-44-2
- Empirical Formula: C15H10O8
- Molecular Weight: 318.24
- MDL number: MFCD00006827
- EINECS: 208-463-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 15:53:33
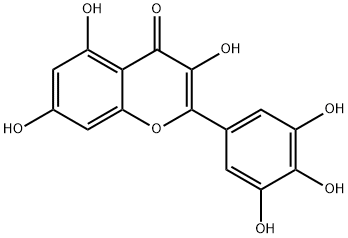
What is Myricetin?
Description
Myricetin is a flavonoid compound found in many fruits and vegetables, including red wine, that acts as a powerful antioxidant. Myricetin inhibits TBARS formation with an IC50 value of 6.34 and at 20 μM, blocks oxLDL uptake by U937-derived macrophages, reducing CD36 expression. Myricetin demonstrates potent chemopreventative potential by binding JAK1/STAT3 to inhibit the neoplastic transformation of murine JB6 P+ cells and inhibiting MEK1 kinase activity.
Chemical properties
The substance is a brown-yellow to brown-green crystalline powder. It can dissolve in methanol, ethanol, acetone, and ethyl acetate, and has a low solubility in water. It is not soluble in chloroform and petroleum ether. This compound is derived from the fruit of Myrica rubra (Lour.) Sieb. et Zucc. and belongs to the Populus family.
The Uses of Myricetin
Myricetin is used as an antioxidant. It is a cell-permeable flavonoid used in inflammatory, diabetes, and cancer studies. It is effective in protecting neurons against oxidative stresses. Further, it inhibits yeast alfa-glucosidase,1 glyoxalase I in vitro and bovine milk xanthine oxidase.
What are the applications of Application
Myricetin is a cell-permeable, ATP-competitive inhibitor of various casein kinases and protein kinases.
Definition
ChEBI: Myricetin is a hexahydroxyflavone that is flavone substituted by hydroxy groups at positions 3, 3', 4', 5, 5' and 7. It has been isolated from the leaves of Myrica rubra and other plants. It has a role as a cyclooxygenase 1 inhibitor, an antineoplastic agent, an antioxidant, a plant metabolite, a food component, a hypoglycemic agent and a geroprotector. It is a hexahydroxyflavone and a 7-hydroxyflavonol. It is a conjugate acid of a myricetin(1-).
General Description
Myricetin is a novel flavanoid compound useful as chemotherapeutic, chemopreventive, and antiangiogenic agent. It also acts as an antioxidant, anti-inflammatory, and anti-cancer agent, and can inhibit the activity of reverse transcriptase.
Biochem/physiol Actions
Myricetin exerts anti-oxidant effects, and anti-inflammatory effects by regulating multiple signal pathways. It also displays anti-diabetic and hepatoprotective effects. Myricetin strongly inhibits yeast α-glucosidase, glyoxalase I in vitro, and bovine milk xanthine oxidase. It also promotes complex formation between DNA and both topoisomerase I and II, an effect that may have implications in cancer chemotherapy. Myricetin also has various nutraceuticals values and therapeutic effects.
Purification Methods
Myricetin (Cannabiscetin, 3,3',4',5,5',7-hexahydroxyflavone) [529-44-2] M 318.2, m > 3 0 0o, 357o(dec) (polyphenolic pKEst~8-11). Recrystallise myricetin from aqueous EtOH (m 357o dec, as monohydrate) or Me2CO (m 350o dec, with one mol of Me2CO) as yellow crystals. It is almost insoluble in CHCl3 and AcOH. The hexaacetate has m 213o. [Hergert J Org Chem 21 534 1956, Spada & Cameroni Gazzetta 86 965, 975 1956, Kalff & Robinson J Chem Soc 127 181 1925, Beilstein 18/5 V 670.]
Properties of Myricetin
| Melting point: | >300 °C(lit.) |
| Boiling point: | 377.41°C (rough estimate) |
| Density | 1.4222 (rough estimate) |
| refractive index | 1.4395 (estimate) |
| storage temp. | 2-8°C |
| solubility | ethanol: soluble10mg/mL, clear to very faintly turbid, yellow to very deep greenish-yellow |
| form | crystalline |
| pka | 6.30±0.40(Predicted) |
| color | Yellowish Brown |
| Water Solubility | Soluble in dimethyl sulfoxide,dimethyl formamide and ethanol. Insoluble in water. |
| Merck | 14,6332 |
| BRN | 332331 |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 529-44-2(CAS DataBase Reference) |
Safety information for Myricetin
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Myricetin
| InChIKey | IKMDFBPHZNJCSN-UHFFFAOYSA-N |
Myricetin manufacturer
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3,4-Dibenzyloxybenzaldehyde 4-Hydrazinobenzoic acid 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 4-HYDROXY BENZYL ALCOHOL 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 3-NITRO-2-METHYL ANILINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
 Myricetin CAS 529-44-2View Details
Myricetin CAS 529-44-2View Details
529-44-2 -
 Myricetin 98% (HPLC) CAS 529-44-2View Details
Myricetin 98% (HPLC) CAS 529-44-2View Details
529-44-2 -
 Myricetin, ≥97% CAS 529-44-2View Details
Myricetin, ≥97% CAS 529-44-2View Details
529-44-2 -
 Myricetin CAS 529-44-2View Details
Myricetin CAS 529-44-2View Details
529-44-2 -
 Myricetin CAS 529-44-2View Details
Myricetin CAS 529-44-2View Details
529-44-2 -
 Myricetin CAS 529-44-2View Details
Myricetin CAS 529-44-2View Details
529-44-2 -
 Myricetin CAS 529-44-2View Details
Myricetin CAS 529-44-2View Details
529-44-2 -
 Myricetin CAS 529-44-2View Details
Myricetin CAS 529-44-2View Details
529-44-2