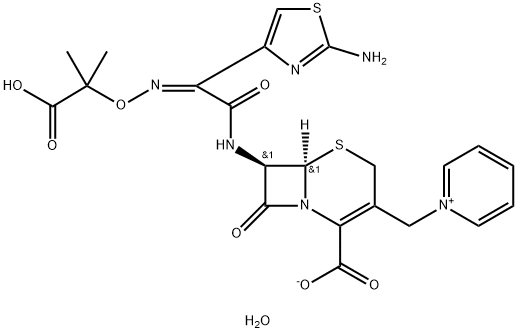
Cefroxadine
- CAS NO.:51762-05-1
- Empirical Formula: C16H19N3O5S
- Molecular Weight: 365.4
- MDL number: MFCD01940013
- EINECS: 257-391-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-04 17:34:39
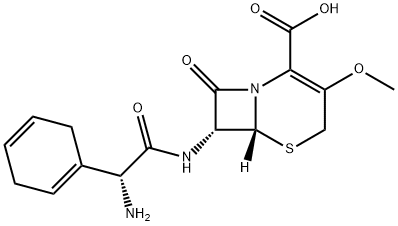
What is Cefroxadine?
Description
Cefroxadine was synthesized by Ciba-Geigy in 1972. A methoxyl group replaced the methyl group of cephradine at the 3 position of the cephem nucleus. Cefroxadine shows stronger activities than cephalexin, especially bactericidal and bacteriolytic activities, and it has better oral absorption that is less affected by a recent meal. Cefroxadine shows less renal toxicity than cephalexin in toxicological studies using animals.
Originator
Oraspor,Ciba Geigy,Switz.,1981
The Uses of Cefroxadine
Antibacterial.
Definition
ChEBI: Cefroxadine is a first-generation cephalosporin antibiotic having methoxy and [(2R)-2-amino-2-(cyclohexa-1,4-dien-1-yl)acetyl]amino side groups located at positions 3 and 7 respectively.
Manufacturing Process
A suspension of 30.64 g (0.2 mol) of D-α-amino-α-(1,4-cyclohexadienyl)-
acetic acid in 600 ml of methylene chloride is cooled under a stream of argon
to 6°C, whereupon hydrogen chloride is passed in for about 30 minutes until
the mixture is saturated. Phosphorpentachloride (62.4 g, 0.3 mol) is added in
two portions. The mixture is stirred for 2 hours at 6°C to 8°C. The colorless
precipitate is filtered off under nitrogen and exclusion of moisture, washed
with methylene chloride and dried for 18 hours at 0.05 mm Hg at room
temperature to give D-α-amino-α-(1,4-cyclohexadienyl)-acetylchloride
hydrochloride in form of colorless crystals.
A suspension of 37.3 g (0.1 mol) of 7β-amino-3-methoxy-3-cephem-4-
carboxylic acid hydrochloride dioxanate in 500 ml methylene chloride is stirred
for 15 minutes at room temparature under an argon atmosphere and treated
with 57.2 ml (0.23 mol) of bis-(trimethylsilyl)acetamide. After 45 minutes the
faintly yellow slightly turbid solution is cooled to 0°C and treated within 10
minutes with 31.29 (0.15 mol) of D-α-amino-α-(1,4-cyclohexadienyl)-acetyl
chloride hydrochloride. Thirty minutes thereafter 15 ml (about 0.21 mol) of
propylene oxide is added and the mixture is further stirred for 1 hour at 0°C:
A cooled mixture of 20 ml of absolute methanol in 200 ml of methylene
chloride is added within 30 minutes, after another 30 minutes the precipitate
is filtered off under exclusion of moisture, washed with methylene chloride
and dried under reduced pressure at room temperature. The obtained
hygroscopic crystals of the hydrochloride of 7β-[D-α-(1,4-cyclohexadienyl)-
acetylamino]-3-methoxy-3-cephem-4-carboxylic acid are stirred into 200 ml of
ice water and the milky solution treated with about 66 ml of cold 2 N sodium
hydroxide solution until pH 3.5 is reached. The solution is clarified by filtration
through diatomaceous earth, washed with ice water, cooled to 0°C and treated
with 20 ml of 2 N sodium hydroxide solution until pH 5.7 is reached. A second
filtration through a glass filter frit results in a clear solution which is treated
with acetone (800 ml) at 0°C. The crystals are filtered washed with
acetone:water (2:1), acetone and diethyl ether and dried for 20 hours at
room temperature and 0.05 mm Hg to give the 7β-[D-α-amino-α-(1,4-
cyclohexadienyl)-acetylamino]-3-methoxy-3-cephem-4-carboxylic acid
dihydrate.
Therapeutic Function
Antibacterial
Antimicrobial activity
Cefroxadine is closely related to cefradine, the structure differing only by the presence of a methoxy group replacing methyl at the C-3 position. The antimicrobial spectrum is identical to that of cefradine and cefalexin. A dose of 1 g as film-coated tablets produced mean peak plasma levels of 25 mg/L at 1 h. Absorption is depressed and delayed by administration with food. The plasma elimination half-life is 0.8 h, rising to 40 h in end-stage renal failure and falling to 3.4 h during hemodialysis. Around 85% of an oral dose is excreted unchanged in the urine. It is available in Japan.
Properties of Cefroxadine
| Melting point: | 170° (dec) |
| Boiling point: | 251°C (rough estimate) |
| alpha | D20 +87° (c = 1.093 in 0.1N HCl) |
| Density | 1.3163 (rough estimate) |
| refractive index | 1.6390 (estimate) |
| pka | pKa 3.30±0.02(H2O t=35.0 I=0.00)(Approximate) |
Safety information for Cefroxadine
Computed Descriptors for Cefroxadine
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran
You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4