Cefpiramide acid
- CAS NO.:70797-11-4
- Empirical Formula: C25H24N8O7S2
- Molecular Weight: 612.64
- MDL number: MFCD00864893
- EINECS: 1308068-626-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 20:33:22
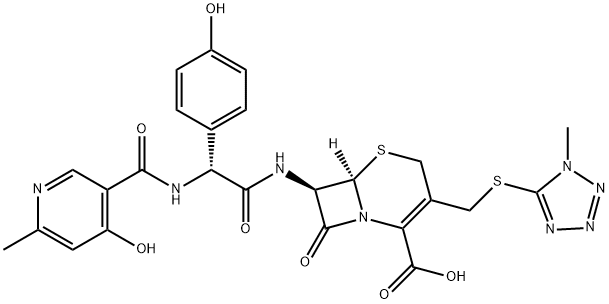
What is Cefpiramide acid?
Absorption
Rapidly absorbed following intramuscular injection.
Toxicity
Adverse effects following overdosage include nausea, vomiting, epigastric distress, diarrhea, and convulsions.
The Uses of Cefpiramide acid
Antibacterial.
The Uses of Cefpiramide acid
Cefpiramide is used as a β-lactam antibiotic.
Indications
For treatment of severe infections caused by susceptible bacteria such as P. aeruginosa.
Background
Cefpiramide is a third-generation cephalosporin antibiotic.
What are the applications of Application
Cefpiramide is β-lactam antibiotic
Definition
ChEBI: A third-generation cephalosporin antibiotic with [(1-methyl-1H-tetrazol-5-yl)sulfanyl]methyl and (R)-2-{[(4-hydroxy-6-methylpyridin-3-yl)carbonyl]amino}-2-(4-hydroxyphenyl)acetamido groups at positions 3 and 7, respectiv ly, of the cephem skeleton. It has a broad spectrum of antibacterial activity.
Antimicrobial activity
A semisynthetic parenteral cephalosporin. It exhibits a broad
range of activity, which includes Ps. aeruginosa, though the overall activity is rather modest. It is moderately
stable to most β-lactamases but less so than ceftazidime or
cefpirome.
In volunteers given 0.5 or 1 g by intravenous bolus, the mean
plasma concentration shortly after injection was around 150 or
300 mg/L, respectively. There was no accumulation when the
same doses were repeated every 12 h for 11 doses. It is highly
bound to plasma protein (c. 95%). The mean plasma half-life
is around 5 h. Less than one-quarter of the dose appears in the
urine over 24 h; the rest is excreted in bile and high concentrations
are found in feces. Renal impairment has little effect on
elimination in patients with normal liver function.
Diarrhea may be associated with marked suppression of
gut flora resulting from biliary excretion of the drug. The molecule
includes a C-3 methylthiotetrazole side chain and side
effects associated with that substituent are to be expected.
It is available in Japan.
Pharmacokinetics
Cefpiramide is a cephalosporin active against Pseudomonas aeruginosa. It has a broad spectrum of antibacterial activity. Cefpiramide works by inhibiting bacterial cell wall biosynthesis. The plasma half-lives of cefpiramide in rabbits, dogs, and rhesus monkeys were much longer than those of cefoperazone and cefazolin.
Metabolism
Not Available
Properties of Cefpiramide acid
| Melting point: | 213-215° (dec) |
| Density | 1.75±0.1 g/cm3(Predicted) |
| storage temp. | -20°C Freezer, Under Inert Atmosphere |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 2.62±0.50(Predicted) |
| color | White |
Safety information for Cefpiramide acid
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H317:Sensitisation, Skin H360:Reproductive toxicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P272:Contaminated work clothing should not be allowed out of the workplace. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P308+P313:IF exposed or concerned: Get medical advice/attention. P333+P313:IF SKIN irritation or rash occurs: Get medical advice/attention. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for Cefpiramide acid
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 Cefpiramide 98% (HPLC) CAS 70797-11-4View Details
Cefpiramide 98% (HPLC) CAS 70797-11-4View Details
70797-11-4 -
 Cefpiramide 95.00% CAS 70797-11-4View Details
Cefpiramide 95.00% CAS 70797-11-4View Details
70797-11-4 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1