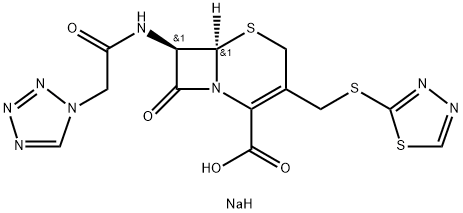
Cefapirin sodium
Synonym(s):3-[(Acetyloxy)methyl]-8-oxo-7-{(4-pyridinylthio)acetyl]amino}-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid monosodium salt;Cefapirin sodium;Cephapirin sodium
- CAS NO.:24356-60-3
- Empirical Formula: C17H18N3NaO6S2
- Molecular Weight: 447.46
- MDL number: MFCD07793329
- EINECS: 246-194-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
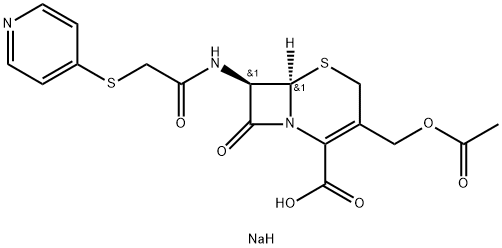
What is Cefapirin sodium?
Description
Cephapirin sodium (Cefapirin sodium), a semisynthetic cephalosporin antibiotic, is bactericidal against strains of gram-positive and gram-negative bacteria.
Cephapirin is closely resembles cephalothin in chemical and pharmacokinetic properties. Cephapirin, have cephalosporanic acid core with the acetyloxymethyl group at the 3rd position and having IUPAC name (6R,7R)-3-(Acetoxymethy)-8- oxo-7-{[(pyridin-4-ylsulfanyl)acetyl]amino}-5-thia- 1-azabicyclo [4.2.0] oct-2-ene-2-carboxylic acid. It is unstable in acid and must be administered parenteral. It has similar mechanism as other cephalosporins. It is marketed under the trade name Cefadyl. It is effective against a wide variety of gram-positive and gramnegative bacteria; used as the sodium salt. Among the most serious adverse reactions of cefapirin, that is, neutropenia, leukopenia, anemia, bone marrowdepression, and allergic reactions, it has been discontinued in the United States (Wiesner, 1972).
Chemical properties
White or pale yellow powder.
Originator
Cefadyl,Bristol,US,1974
The Uses of Cefapirin sodium
Cefapirin sodium is very similar to cephalothin (Bran et al., 1972). Cephapirin is now used almost exclusively in veterinary practice.
What are the applications of Application
Cefapirin sodium is an antibacterial agent
Definition
ChEBI: Cephapirin sodium is the sodium salt of cephapirin. A first-generation cephalosporin antibiotic, it is effective against gram-negative and gram-positive organisms. Being more resistant to beta-lactamases than penicillins, it is effective agains most staphylococci, though not methicillin-resistant staphylococci. It has a role as an antibacterial drug. It is a cephalosporin and an organic sodium salt. It contains a cephapirin(1-).
What are the applications of Application
Cephapirin was synthesized by BristolMyers Laboratories in 1970. It shows almost the same in vitro antibacterial activity as cephalothin, but its in vivo effects are slightly greater than those of cephalothin. Like cephalothin, it is metabolized in vivo, and its deacetylated metabolite shows almost the same activity against gram-positive bacteria as cephalothin, but weaker activity against gramnegative bacteria. Cephapirin has been used for therapy of urinary tract infections and osteomyelitis caused by Staphylococcus, Streptococcus, and Escherichia coli.
brand name
Cefadyl (Apothecon), ToDAY
Therapeutic Function
Antibacterial
Synthesis
Cephapirin, (6R-trans)-3-[(acetyloxy)methyl]-8-oxo-7-[[(4-pyridinylthio)
acetyl]amino]-5-thia-1-azabicyclo[4.2.0]oct-2-en-2-carboxylic acid (32.1.2.4), is synthesized by acylating 7-aminocephalosporanic acid with 4-pyridylthioacetic acid chloride
(32.1.2.3), which is synthesized by reacting 4-chloropyridine with mercaptoacetic acid in
the presence of a base, forming 4-pyridylthioacetic acid (32.1.22), and further transforming the resulting acid to the acid chloride by reacting it with phosphorous pentachloride.
An alternative way of making cephapirin is the acylation of 7-aminocephalosporanic acid
by bromoacetyl bromide, which gives a bromoacetyl derivative (32.1.2.5), and which is
then reacted with 4-mercaptopyridine in the presence of triethylamine, forming the desired
cephapirin (32.1.2.4).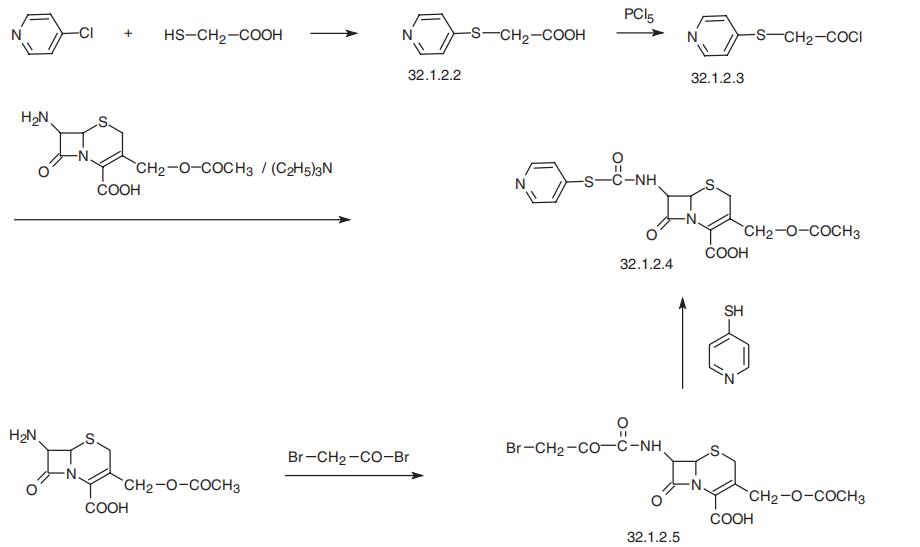
Properties of Cefapirin sodium
| Melting point: | >177°C (dec.) |
| alpha | +152~+170゜(25℃/D)(c=2,H2O)(calculated on the dehydrous basis) |
| storage temp. | Inert atmosphere,2-8°C |
| solubility | Soluble in water, practically insoluble in methylene chloride. |
| form | powder |
| pka | pKa 2.15 (Uncertain) |
| color | Light Beige to Beige |
| CAS DataBase Reference | 24356-60-3(CAS DataBase Reference) |
Safety information for Cefapirin sodium
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H317:Sensitisation, Skin H319:Serious eye damage/eye irritation H334:Sensitisation, respiratory H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P284:Wear respiratory protection. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P342+P311:IF experiencing respiratory symptoms: call a POISON CENTER or doctor/physician. |
Computed Descriptors for Cefapirin sodium
| InChIKey | VGEOUKPOQQEQSX-OALZAMAHSA-M |
Cefapirin sodium manufacturer
Nectar Lifesciences Ltd
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 24356-60-3 Cephapirin sodium 98%View Details
24356-60-3 Cephapirin sodium 98%View Details
24356-60-3 -
 Cefapirin sodium 95% CAS 24356-60-3View Details
Cefapirin sodium 95% CAS 24356-60-3View Details
24356-60-3 -
 Cephapirin sodium CAS 24356-60-3View Details
Cephapirin sodium CAS 24356-60-3View Details
24356-60-3 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1