Cefotiam
- CAS NO.:61622-34-2
- Empirical Formula: C18H23N9O4S3
- Molecular Weight: 525.63
- MDL number: MFCD00865087
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 20:33:22
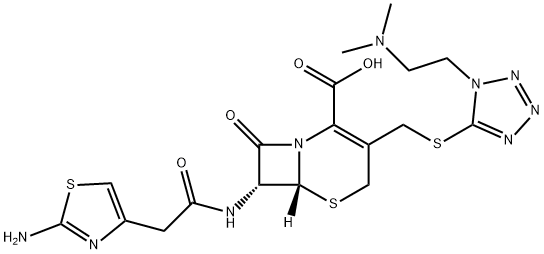
What is Cefotiam?
Absorption
Rapidly absorbed following intramuscular injection. Bioavailability is 60% following intramuscular injection.
Toxicity
Adverse effects following overdosage include nausea, vomiting, epigastric distress, diarrhea, and convulsions.
Originator
Cefotiam,Qingdao Ftz United International Inc.
The Uses of Cefotiam
Antibacterial
Background
One of the cephalosporins that has a broad spectrum of activity against both gram-positive and gram-negative microorganisms.
Indications
For treatment of severe infections caused by susceptible bacteria.
Definition
ChEBI: A cephalosporin with ({1-[2-(dimethylamino)ethyl]-1H-tetrazol-5-yl}sulfanyl)methyl and (2-amino-1,3-thiazol-4-yl)acetamido substituents at positions 3 and 7, respectively, of the cephem skeleton. A third generation beta-lactam cephalospo in antibiotic, it is active against a broad spectrum of both Gram positive and Gram negative bacteria.
Manufacturing Process
2 mmol of 7-acetamindocephalosporinic acid in phosphate buffer (pH 7), 2
mmol of 1-(2-dimethylaminoethyl-1H-tetrazol-5-thiol and 2.2 mmol of sodium
hydrogen carbonate are mixed and resulting solution was stirred at 60°-65°C
for 16 hours. After cooling the mixture was adjusted pH 3.6 and hydrochloride
of NH2OH was added to give 7-amino-3-[2-(2-dimethylaminoethyl)-2H-tetrazol-5-ylsulfanylmethyl]-8-oxo-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-
carboxylic acid.
0.4 g (2 mmol) of 2-(2-aminothiazol-4-yl)acetic acid hydrochloride in 10 ml of
dimethylformamide are dissolved, 0.25 g (2.2 mmol) of N-hydroxysuccinimide
and 0.412 g (2 mmol) of dicyclohexylcarbodiimide and the solution is allowed
to stand at room temperature for 3 hours. The reaction mixture is subjected
to filtration under suction to remove the precipitate of N,N'-dicyclohexylurea.
The filtrate is added at a stroke to a solution of 2 mmol of above prepared 7-
amino-3-[2-(2-dimethylaminoethyl)-2H-tetrazol-5-ylsulfanylmethyl]-8-oxo-5-
thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 0.404 g (4 mmol) of
triethylamine in 20 ml of dichloromethane and the mixed solution is stirred for
24 hours at room temperature. The solvent is distilled off under reduced
pressure and the residue is adjusted pH from to 6 to 7 by adding a 10%
aqueous solution of sodium hydrogen carbonate. The resultant solution is
chromatographed on column of polystyrene resin (Amberlite XAD-2) and
developed with water. The fractions containing the desired product are pooled
and freeze-dried to obtain the title product.
brand name
Ceradon (Takeda).
Therapeutic Function
Antibiotic
Antimicrobial activity
A semisynthetic cephalosporin formulated as the dihydrochloride
for injection and as a prodrug ester, cefotiam hexetil, for
oral administration. Activity is similar to that of cefuroxime,
but it is somewhat more active against a range of enterobacteria
.
A 30-min intravenous infusion of the dihydrochloride
produces a peak serum concentration of 35 mg/L; the corresponding
concentration after a 1 g intramuscular dose is
17 mg/L. Oral absorption of the hexetil ester is around 65%.
Food delays absorption of the ester. The plasma half-life is
0.6–1.1 h. Around 40% is bound to plasma protein.
Urinary excretion is almost complete 4 h after the end
of intravenous infusion, but only 50–67% is recovered
unchanged; there is substantial non-renal elimination and
some evidence of saturation of renal tubular excretion at
doses above 2 g. In anuria the plasma elimination half-life
rises to 13 h and plasma and renal clearances parallel creatinine
clearance. A small amount is excreted in bile. In patients
with cholelithiasis given 0.5 or 1 g intravenously, mean concentrations
in gallbladder bile and gallbladder wall 30 min
after the dose were around 17 and 32 mg/L, respectively. In
patients with normal liver function, hepatic bile concentrations
can exceed 1 g/L.
It is generally well tolerated and has been used successfully
to treat lower respiratory infections, skin and soft-tissue infection.
It is not widely used, but is available in Japan and some
other countries.
Pharmacokinetics
Cefotiam is a third generation beta-lactam cephalosporin antibiotic that works by inhibiting bacterial cell wall biosynthesis. It is a broad spectrum antibiotic that is effective against Gram positive and Gram negative bacteria.
Metabolism
Not Available
Properties of Cefotiam
| Boiling point: | 843℃ |
| Density | 1.80±0.1 g/cm3(Predicted) |
| RTECS | XI0366000 |
| Flash point: | >110°(230°F) |
| storage temp. | 2-8°C |
| pka | 2.57±0.50(Predicted) |
| Water Solubility | Soluble |
| CAS DataBase Reference | 61622-34-2(CAS DataBase Reference) |
Safety information for Cefotiam
Computed Descriptors for Cefotiam
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran




You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1