3-Dimethylaminopropylamine
Synonym(s):N,N-Dimethyl-1,3-diaminopropane;N,N-Dimethyl-1,3-propanediamine;3-Dimethylaminopropylamine, N,N-Dimethyl-1,3-propanediamine, N,N-Dimethyl-1,3-diaminopropane;N,N-Dimethyltrimethylenediamine
- CAS NO.:109-55-7
- Empirical Formula: C5H14N2
- Molecular Weight: 102.18
- MDL number: MFCD00008216
- EINECS: 203-680-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
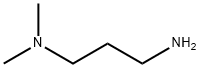
What is 3-Dimethylaminopropylamine?
Description
Dimethylaminopropylamine is an aliphatic amine present in amphoteric surfactants such as liquid soaps and shampoos. It is present as an impurity responsible for allergy from cocamidopropylbetaine. It is structurally similar to diethylaminopropylamine. It is also used as a curing agent for epoxy res ins and as an organic intermediate in chemical syntheses (ion exchangers, additives for flocculants, cosmetics and fuel additives, dyes and pesticides).
Chemical properties
clear liquid. soluble in water and organic solvents.
The Uses of 3-Dimethylaminopropylamine
N,N-Dimethyl-1,3-propylenediamine is a useful catalyst for Knoevenagel condensation. It is also a useful hardening agent for expoxy resin.
The Uses of 3-Dimethylaminopropylamine
3-(Dimethylamino)propylamine is an intermediate substance in the synthesis of alkylamidopropyldimethylamines/alkylamidobetaines; found as an impurity in cosmetic surfactants present in e.g. shampoos; hardener of epoxy resins; additive in fue!, dyes, pesticides and binding agents; in the production of ion-exchangers.
The Uses of 3-Dimethylaminopropylamine
Useful for cleavage of N-alkylphthalimide derivatives to give primary amines, as an alternative to methyl hydrazine. DMAPA is also used directly as a hardener in epoxy resins in the plastics industry, as a cross linking agent for cellulose fibers in the paper industry and as an anti-shriking agent for leather. It is used as an intermediate in the production of binding agents, ion-exchange materials, flocculating agents (water treatment), cosmetic agents, washing and cleaning agents (betaines), additive for petrol and other fuels, polyurethane fibers and lubricants, dyes, agrochemicals, agents used in the photographic and textile industries.
Production Methods
3-Dimethylaminopropylamine is produced by addition of dimethylamine to acrylonitrile and subsequent hydrogenation in the presence of ammonia.
General Description
Dimethylaminopropylamine is a colorless liquid. (NTP, 1992)
Air & Water Reactions
Highly flammable. Temperature sensitive and possibly sensitive to air. Water soluble.
Reactivity Profile
3-Dimethylaminopropylamine neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen may be generated in combination with strong reducing agents, such as hydrides.
Fire Hazard
3-Dimethylaminopropylamine is flammable.
Safety Profile
Moderately toxic by ingestion and skin contact. A skin and eye irritant. Very flammable when exposed to heat, flame, or oxidmers. Reaction with 1,2dichloroethane produces explosive acetylene gas. This and other amines igmte on contact with cellulose nitrate of hgh surface area. To fight fire, use alcohol foam, CO2, dry chemical. When heated to decomposition it emits toxic fumes of NO,. See also AMINES
Toxicology
There are indications of contact and possibly respiratory allergy in relation to occupational exposure. Less than 0.9 ppm of 3-dimethylaminopropylamine in the workplace air may adversely affect respiratory function. The skin-sensitizing potential was verified in an animal study. In an oral 28-d study with rats (gavage, 7 times per week.), clinical symptoms including pulmonary and cardiac failure were noted at 250 mg kg -1 d -1, but no treatment-related effects at a level of 50 mg kg -1 d -1.
Properties of 3-Dimethylaminopropylamine
| Melting point: | -60 °C |
| Boiling point: | 133 °C(lit.) |
| Density | 0.812 g/mL at 25 °C(lit.) |
| vapor density | 3.6 (vs air) |
| vapor pressure | 5 mm Hg ( 20 °C) |
| refractive index | n |
| Flash point: | 90 °F |
| storage temp. | Store below +30°C. |
| form | Liquid |
| pka | 10.13±0.10(Predicted) |
| color | Clear |
| PH | 12.7 (100g/l, H2O, 20℃) |
| Odor | ammoniacal odor |
| explosive limit | 2.3-12.35%(V) |
| Water Solubility | SOLUBLE |
| FreezingPoint | -70℃ |
| Sensitive | Air Sensitive |
| BRN | 605293 |
| CAS DataBase Reference | 109-55-7(CAS DataBase Reference) |
| NIST Chemistry Reference | 1,3-Propanediamine, N,N-dimethyl-(109-55-7) |
| EPA Substance Registry System | 3-(Dimethylamino)propylamine (109-55-7) |
Safety information for 3-Dimethylaminopropylamine
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H226:Flammable liquids H314:Skin corrosion/irritation H317:Sensitisation, Skin H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P333+P313:IF SKIN irritation or rash occurs: Get medical advice/attention. P403+P235:Store in a well-ventilated place. Keep cool. |
Computed Descriptors for 3-Dimethylaminopropylamine
| InChIKey | IUNMPGNGSSIWFP-UHFFFAOYSA-N |
3-Dimethylaminopropylamine manufacturer
Gomati Impex Private Limited
Prakash Chemicals International Private Limited
Triveni Interchem Private Limited (Group Of Triveni Chemicals)
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran




You may like
-
 3-Dimethylamino-1-propylamine 98%View Details
3-Dimethylamino-1-propylamine 98%View Details -
 3-Dimethylaminopropylamine CAS 109-55-7View Details
3-Dimethylaminopropylamine CAS 109-55-7View Details
109-55-7 -
 N,N-Dimethyl-1,3-propanediamine CAS 109-55-7View Details
N,N-Dimethyl-1,3-propanediamine CAS 109-55-7View Details
109-55-7 -
 Dimethylaminopropylamine CASView Details
Dimethylaminopropylamine CASView Details -
 N,N-Dimethyl-1,3-propanediamine 95% CAS 109-55-7View Details
N,N-Dimethyl-1,3-propanediamine 95% CAS 109-55-7View Details
109-55-7 -
 Dimethyl Amino Propyl Amine, Liquid, Purity: 99%View Details
Dimethyl Amino Propyl Amine, Liquid, Purity: 99%View Details
109-55-7 -
 Dimethylaminopropylamine (DMAPA) C5H14N2, Purity: 98%, LiquidView Details
Dimethylaminopropylamine (DMAPA) C5H14N2, Purity: 98%, LiquidView Details
109-55-7 -
 Di Methyl Amino Propyl Amine (Dmapa) CAS 109-55-7, For Industrial, Packaging Size: 165View Details
Di Methyl Amino Propyl Amine (Dmapa) CAS 109-55-7, For Industrial, Packaging Size: 165View Details
109-55-7
