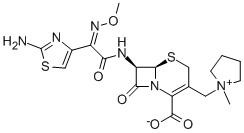
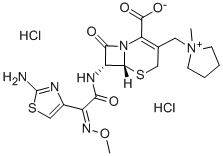
Cefepime
- CAS NO.:88040-23-7
- Empirical Formula: C19H24N6O5S2
- Molecular Weight: 480.56
- MDL number: MFCD00864890
- EINECS: 643-019-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-03 16:17:04
What is Cefepime?
Absorption
Healthy adult male volunteers (n=9) given a single intravenous infusion of 500 mg, 1 g, and 2 g of cefepime had a corresponding Cmax of 39.1, 81.7 and 163.9 μg/mL, and a corresponding AUC of 70.8, 148.5 and 284.8 h?μg/mL. On the other hand, healthy adult male volunteers given a single intramuscular infusion of 500 mg, 1 g, and 2 g of cefepime had a corresponding Cmax of 13.9, 29.6 and 57.5 μg/mL, a corresponding AUC of 60, 137 and 262 h?μg/mL, and a corresponding Tmax of 1.4, 1.6 and 1.5 h.
A study in healthy adult male volunteers (n=7) that received clinically relevant doses for 9 days suggests that cefepime is not accumulated in the body. Between 250 mg and 2 g, cefepime follows a linear pharmacokinetic model, and the absolute bioavailability of cefepime in pediatric patients (n=8) given an intramuscular dose of 50 mg/kg was 82.3%.
Toxicity
Patients who receive a cefepime overdose should be carefully observed and given supportive treatment. In case of renal insufficiency, peritoneal dialysis should not be performed. Instead, hemodialysis is recommended to aid in the removal of cefepime from the body. Some of the symptoms of a cefepime overdose are encephalopathy (disturbance of consciousness including confusion, hallucinations, stupor, and coma), myoclonus, seizures, neuromuscular excitability and nonconvulsive status epilepticus. In vivo carcinogenicity studies for cefepime have not been performed. In chromosomal aberration studies, this antibiotic was positive for clastogenicity in primary human lymphocytes, but negative in Chinese hamster ovary cells. Cefepime does not exhibit genotoxic effects in in vitro assays, and in vivo assessments of clastogenicity are negative. In rats given up to 1000 mg/kg/day (1.6 times the recommended maximum human dose), cefepime did not have negative effects on fertility.
Description
Cefepime is a new fourth-generation parenteral cephalosporine antibiotic launched in 1993 in Sweden and France. Cefepime has broad spectrum antimicrobial activity against Staphylococcus, Strepfococcus, Pseudomonas, and the Enterobacteriaceae, including many bacterial isolates that are resistant to commonly used ceftazidime and cefotaxime. Its efficacy has been demonstrated in the treatment of lower respiratory tract infections especially pneumonia, intra-abdominal and urinary tract infections, skin and soft tissue infections, chronic osteomyelitis and in prophylaxis of biliary tract and prostate infections. It is well tolerated by patients and is reported to exhibit no significant drug interactions.
Chemical properties
colorless Powder
Originator
Bristol-Myers Squibb (U.S.A.)
The Uses of Cefepime
Cefepime is used for bacterial infections caused by microorganisms that are sensitive to drugs in septicemia, bacteriemia, complicated infections of the upper and lower sections of the urinary system, pneumonia, pulmonary abscesses, emphysema of the pleura, fever in patients with neutropenia, and infected skin and soft tissue wounds. Synonyms of this drug are maxipime, cepim, cepimex, and others.
Indications
Cefepime is indicated for the treatment of pneumonia caused by susceptible bacteria, and for empiric therapy for febrile neutropenic patients. Cefepime is also indicated for the treatment of uncomplicated and complicated urinary tract infections (including pyelonephritis), uncomplicated skin and skin structure infections, and complicated intra-abdominal infections (used in combination with metronidazole) in adults caused by susceptible bacteria.
What are the applications of Application
Cefepime is a broad-spectrum cephalosporin antibiotic against gram-positive and gram-negative bacteria
Background
Cefepime is a fourth-generation cephalosporin antibiotic developed in 1994. Cefepime is active against Gram-positive and Gram-negative bacteria, and has greater activity against both compared to third-generation antibiotics. Cefepime is normally used to treat severe nosocomial pneumonia and infections caused by multi-resistant microorganisms such as Pseudomonas aeruginosa, and is also indicated for the empirical treatment of febrile neutropenia. The popularity of its third-generation predecessors, its clinical efficacy, and the high prevalence of multidrug-resistant bacteria might be some of the factors leading to an increase in the use of cefepime. The activity of cefepime against Enterobacteriaceae, Pseudomonas aeruginosa, and Staphylococcus aureus is due to its high stability toward beta-lactamases. In general, cefepime seems to be well tolerated; however, patients treated with this antibiotic, especially those with renal impairment, may develop neurotoxicity.
Definition
ChEBI: Cefepime is a cephalosporin bearing (1-methylpyrrolidinium-1-yl)methyl and (2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-(methoxyimino)acetamido groups at positions 3 and 7, respectively, of the cephem skeleton. It has a role as an antibacterial drug. It is a cephalosporin and an oxime O-ether. It is a conjugate base of a cefepime(1+).
Manufacturing Process
A mixture of ethyl (Z)-2-hydroxyimino-2-(2-tritylaminothiazol-4-yl) acetate (5
g, 10.9 mmoles), methyliodide (2.04 ml, 32.8 mmoles) and K2CO3 (4.54 g,
32.8 mmoles) in dry DMSO (100 ml) was stirred at room temperature
overnight and then poured into water (250 ml). The precipitate was collected,
washed with water and dried to give 2-methoxyimino-2-(2-tritylaminothiazol-
4-yl) acetate (1.15 g, melting point 115°C (dec.))
Ethyl (Z)-2-hydroxyimino-2-(2-tritylaminothiazol-4-yl) acetate (6 g, 12.7
mmol) in ethanol was treated with 2 N NaOH (12.7 ml) at room temperature
overnight. The mixture was adjusted to pH 8 by the addition of powdered dry
ice and the solvent evaporated. The residue was dissolved in water (100 ml)
and was added to the solution which was acidified with 1 N HCl to pH 2 and
then extracted with ethyl acetate. Extract was evaporated, the residue was
crystallized from ethyl acetate-hexane to afford ethyl (Z)-2-hydroxyimino-2-
(2-tritylaminothiazol-4-yl)acetic acid (5.56 g, melting point 138-143°C (dec.)).
To a suspension of phosphate buffer (pH 7, 162.5 ml) and wheat bran (20 g,
dry) at room temperature was added 7-phenylacetimidocephalosporanic acid
sodium salt (5 g). After 5 hours the suspension was filtered to remove wheat
bran and the filtrate was cooled to 5-10°C, then was added methylene
chloride (32 ml) and 0.5 M solution of diphenyldiazomethane in methylene
chloride (24 ml). The pH was then adjusted to 3.0 with 28% phosphoric acid.
After 1 hour the mixture was allowed to rise to 20°C. Heptane was slowly
added (56 ml) and was recovered benzhydryl 3-hydroxymethyl-7-
phenylacetamido-3-cephem-4-carboxylate (3.0 g, 50%).
The mixture of PCl5(8.3 g) and pyridine (3.2 g) in CH2Cl2 was added to
benzhydryl 3-hydroxymethyl-7-phenylacetamido-3-cephem-4-carboxylate (5.1
g) at -40°C. The mixture was stirred at -10°C for 15 minutes and allowed to
stand at -15-10°C for 7 hours. To the solution at -20°C was added propane-
1,3-diol (10 ml) and the mixture was allowed to stand at -20°C for 16 hours and then at room temperature for 20 minutes. The resulting solution was
washed with ice-water and saturated aqueous NaCl (10 ml), dried and
concentrated. The gummy residue (12 g) was dissolved in CHCl3-hexane
(2:1), and subjected to chromatography using silica gel column and the same
solvent as eluant. After evaporation of the solvents was obtained benzhydryl-
7-amino-3-chloromethyl-3-cephem-4-carboxylate (2.1 g, 51%, melting point
>110°C(dec.)).
Benzhydryl 7-amino-3-chloromethyl-3-cephem-4-carboxylate (2.29 g) was
treated with bis(trimethylsilyl)acetamide (4.06 ml) at room temperature for
50 min to give a clear solution. Top the solution was added an acid chloride
solution, which was prepared from (Z)-2-hydroxyimino-2-(2-
tritylaminothiazol-4-yl)acetic acid (2.04 g) and PCl5 (1.15 g) in methylene
chloride (20 ml). The mixture was stirred at room temperature for 30 min,
poured in cold water (200 ml) and extracted with ethyl acetate (100 ml x 3).
After evaporation of the solution was obtained the syrup (4 g) which was
chromatographed on a silica gel column by eluting with 10:1 and 3:1 mixture
of toluene and ethyl acetate successively. After evaporation of the solvents
was obtained benzhydryl 3-chloromethyl-7-[(Z)-2-methoxyimino-2-(2-
tritylaminothiazol-4-yl)acetamido]-3-cephem-4-carboxylate (2.62 g, 68%).
A mixture of the benzhydryl 3-chloromethyl-7-[(Z)-2-methoxyimino-2-(2-
tritylaminothiazol-4-yl)acetamido]-3-cephem-4-carboxylate (1.50 g, 1.79
mmoles) and NaI (1.34 g, 8.93 mmoles) in methyl ethyl ketone (30 ml) was
stirred at room temperature for 1 hour. After evaporation of the solvent the
residue was dissolved in ethyl acetate (100 ml) and washed with water,
aqueous Na2S2O3 and aqueous NaCl, dried and evaporated to give 7-[(Z)-2-
ethoxyimino-2-(2-tritylaminothiazol-4-yl)acetamido]-3-iodomethyl-3-cephem-
4-carboxilate (1.47 g, 89%) as an amorphous powder.
A mixture of 7-[(Z)-2-ethoxyimino-2-(2-tritylaminothiazol-4-yl)acetamido]-3-
iodomethyl-3-cephem-4-carboxilate (4.5 g, 4.83 mmoles) and N-methylpyrrolidine (0.65 ml, 6.28 mmoles) in CH2Cl2 (45 ml) was stirred at
room temperature for 20 min. Ether (300 ml) was added to the mixture to
separate the quaternary salt of the blocked cephalosporin, which was collected
by filtration and treated with 90% trifluoroacetic acid (TFA) (40 ml) at room
temperature for 1 hour. The mixture was then evaporated under reduced
pressure below 20°C. The residue was triturated with ether to give the TFA
salt of 7-[(Z)-2-methoxyimino-2-(2-aminothiazol-4-yl)acetamido]-3-[(1-
methyl-1-pyrrolidinium)methyl]-3-cephem-4-carboxylate (2.40 g), which was
dissolved in methanol (5 ml) and treated with 1 M solution of sodium-2-
ethylhexoate in ethyl acetate (8 ml) at room temperature for 30 min. After
the addition of ethyl acetate (100 ml), the precipitate (1.94 g) formed was
collected by filtration. HPLC analysis showed that the crude product was 7%
pure with a 1:8 ratio of the δ3 isomer to the δ2 isomer. Purification of the
product by HPLC was repeated three times (Lichrosorb RP-18, eluted with 5%
aqueous methanol or 0.01 M ammonium phosphate buffer (pH 7.2 containing
5% of methanol) to give 35 mg (1.5%) of the title product as a colorless
powder of 7-[(Z)-2-methoxyimino-2-(2-aminothiazol-4-yl)acetamido]-3-[(1-
methyl-1-pyrrolidinium)methyl]-3-cephem-4-carboxylate. Estimated purity (by
HPLC) 90%. M.p. 150°C (dec.).
brand name
Maxipime; Axepim
Therapeutic Function
Antibiotic
Antimicrobial activity
Its activity against common pathogens is comparable to that of group 4 cephalosporins, but it is somewhat more active against Ps. aeruginosa. Like cefpirome it has low affinity for the molecular class C cephalosporinases of many Gram-negative rods and is consequently active against most strains of Citrobacter spp., Enterobacter spp., Serratia spp. and Ps. aeruginosa that are resistant to cefotaxime and ceftazidime. It has poor activity against L. monocytogenes and against anaerobic organisms.
Pharmacokinetics
Cefepime is a fourth-generation cephalosporin antibiotic. It is active against Gram-negative bacteria such as Enterobacter spp., Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis and Pseudomonas aeruginosa, and Gram-positive bacteria such as Staphylococcus aureus (methicillin-susceptible isolates only), Streptococcus pneumoniae, Streptococcus pyogenes and Viridans group streptococci. Compared to third-generation cephalosporins, cefepime has an extended Gram-negative coverage. Whereas other cephalosporins are degraded by plasmid- and chromosome-mediated beta-lactamases, cefepime is stable and not significantly hydrolyzed by these enzymes. Cefepime is also a poor inducer of type 1 beta-lactamases and, therefore, a good alternative against bacteria resistant to third-generation cephalosporins.
In animal models of infection, the time that the unbound plasma concentration of cefepime exceeds the minimum inhibitory concentration (MIC) of infecting organisms has been shown to correlate with treatment efficacy. It has been suggested that cefepime can cross the inflamed blood-brain barrier. This, along with its ability to inhibit γ-aminobutyric acid (GABA), could lead to the neurotoxic effects observed in some of the patients treated with cefepime.
Pharmacokinetics
Cmax 2 g intravenous (30-min infusion): c. 160 mg/L end infusion
Plasma half-life: c. 2 h
Volume of distribution: 14–20 L
Plasma protein binding: 10–19%
It is well distributed. Penetration into tissues, including lung,
appears to be similar to that of other aminothiazoyl cephalosporins.
Very low concentrations are achieved in CSF in the
absence of meningeal inflammation. It is secreted in breast
milk.
It is partially metabolized, but 85% of the dose is excreted
unchanged in the urine, achieving a concentration approaching
1 g/L within 4 h of a 1 g intravenous dose. Dosage adjustment
is required in patients with impaired renal function,
but hepatic impairment does not affect the pharmacokinetic
properties.
Clinical Use
Proprietary name: Maxipime.
Preparation: Injection.
Dosage: Adult, i.m., i.v., 1–6 g per day in 2–3 divided doses.
Available in USA, most of Europe and Japan; not available in the UK.
Clinical Use
Cefepime (Maxipime, Axepin) is a parenteral, β-lactamase–resistant cephalosporin that is chemically and microbiologicallysimilar to cefpirome. It also has a broadantibacterial spectrum, with significant activity against bothGram-positive and Gram-negative bacteria, including streptococci,staphylococci, Pseudomonas spp., and theEnterobacteriaceae. It is active against some bacterial isolatesthat are resistant to ceftazidime. The efficacy of cefepimehas been demonstrated in the treatment of urinary tract infections,lower respiratory tract infections, skin and soft tissueinfections, chronic osteomyelitis, and intra-abdominal andbiliary infections. It is excreted in the urine with a half-life of2.1 hours. It is bound minimally to plasma proteins. Cefepimeis also a fourth-generation cephalosporin.
Side Effects
It is used in the treatment of serious infections, particularly those in which resistant Gram-negative pathogens are known or suspected to be involved.
Synthesis
Cefepime, {6R-[6α,7β(Z)]}-1-[(7-{[(2-amino-4-thiazolyl)-(methoxyimino) acetyl]-amino}-2-carboxy-8-oxo-5-thia-1-(azabicyclo[4.2.0]oct-2-en-3-yl)methyl-1- methyl]pyrrolidine chloride (32.1.2.99), is synthesized by a combination of methods described for the synthesis of third-generation cephalosporins, in particular, cefaloridin (32.1.2.79) and ceftazidime (32.1.2.82).
Metabolism
Less than 1% of cefepime is metabolized in the liver. Cefepime is metabolized to N-methylpyrrolidine (NMP), which then undergoes rapid oxidation to form NMP-N-oxide, a more stable compound. NMP-N-oxide is the predominant metabolite of cefepime, while NMP and the 7-epimer of cefepime are minor byproducts. It has been suggested that flavin-containing mixed-function oxygenase mediates the oxidation of NMP to NMP-N-oxide.
Properties of Cefepime
| Melting point: | 150 C |
| form | Solid |
| color | White to light yellow |
| CAS DataBase Reference | 88040-23-7(CAS DataBase Reference) |
Safety information for Cefepime
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Cefepime
| InChIKey | HVFLCNVBZFFHBT-IZVURRFBNA-N |
| SMILES | N12C([C@@H](NC(=O)/C(=N\OC)/C3N=C(N)SC=3)[C@H]1SCC(C[N+]1(C)CCCC1)=C2C([O-])=O)=O |&1:2,16,r| |
Cefepime manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 88040-23-7 Cefepime; CFPM 98%View Details
88040-23-7 Cefepime; CFPM 98%View Details
88040-23-7 -
 88040-23-7 98%View Details
88040-23-7 98%View Details
88040-23-7 -
 Cefepime 99%View Details
Cefepime 99%View Details
88040-23-7 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1