Urethane
Synonym(s):Carbamic acid ethyl ester;Ethyl carbamate;Ethylurethane
- CAS NO.:51-79-6
- Empirical Formula: C3H7NO2
- Molecular Weight: 89.09
- MDL number: MFCD00007966
- EINECS: 200-123-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 13:58:55
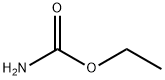
What is Urethane?
Description
Ethyl carbamate occurs as colourless or white columnar crystals or granular powder. It is very soluble in water. With heat, ethyl carbamate undergoes decomposition and emits toxic fumes of carbon monoxide, carbon dioxide, and nitrogen oxides. Ethyl carbamate is used as an intermediate in the synthesis of a number of chemical products, for example, pharmaceuticals, in biochemical research and medicine, and as a solubiliser and co-solvent for pesticides and fumigants. Prior to World War II, ethyl carbamate saw relatively heavy use in the treatment of multiple myeloma before it was found to be toxic, carcinogenic, and largely ineffective. However, due to U.S. FDA regulations, ethyl carbamate has been withdrawn from pharmaceutical use. However, small quantities of ethyl carbamate are also used in laboratories as an anaesthetic for animals.
Chemical properties
Colorless crystals or white powder; odorless, saltpeter-like taste. Solutions neutral to litmus. Soluble in water, alcohol, ether, glycerol, and chloroform; slightly soluble in olive oil. Combustible.
Chemical properties
Urethane is a colorless, almost odorless crystalline solid or powder.
Occurrence
Urethane (ethyl carbamate) occurs as a natural byproduct in fermented products such as wine, liquors, yogurt, beer, bread, olives, cheeses, and soy sauces. Whereas urethane has a known cancer etiology in experimental animals, no such relationship has yet been proven in humans. Alcohol may act by blocking the metabolism of urethane, and thus exert a protective effect in humans consuming alcoholic beverages.
The Uses of Urethane
The primary use of urethane has been as a chemical intermediate in preparation of amino resins (IARC 1974). The process involves a reaction with formaldehyde to give hydroxymethyl derivatives that are used as cross-linking agents in permanent-press textile treatments designed to impart wash-and-wear properties to fabrics. Urethane isalso used as a solubilizer and co-solvent in the manufacture of pesticides, fumigants, and cosmetics, as an intermediate in the manufacture of pharmaceuticals, and in biochemical research (HSDB 2009). Urethane was formerly used as an active ingredient in drugs prescribed for the treatment of neoplastic diseases, as a sclerosing solution for varicose veins, as a hypnotic, and as a topical bactericide. It is also used in veterinary medicine as an anesthetic (IARC 1974). Urethane is produced naturally during many fermentation processes(Zimmerli and Schlatter 1991).
The Uses of Urethane
Naturally occurring contaminant in fermented foods, particularly wine, stone-fruit brandies, and bread. This substance is reasonably anticipated to be a human carcinogen.
The Uses of Urethane
antiinfective
The Uses of Urethane
Intermediate in organic synthesis. In the preparation and modification of amino resins. As solvent, solubilizer and cosolvent for various organic materials. Animal anesthetic in laboratory procedures.
What are the applications of Application
Urethane is a naturally occurring contaminant
Background
Urethane, formerly marketed as an inactive ingredient in Profenil injection, was determined to be carcinogenic and was removed from the Canadian, US, and UK markets in 1963.
Definition
A poisonous flammable organic compound, used in medicine, as a solvent, and as an intermediate in the manufacture of polyurethane resins.
World Health Organization (WHO)
Urethane was formerly used as an antineoplastic agent in the treatment of chronic myeloid leukaemia. It is also a mild hypnotic which has been used as an anaesthetic for veterinary practice. It has been reported to have both a carcinogenic and mutagenic potential. Although urethane continues to be used as an industrial solvent, WHO has no information to suggest that it remains commercially available in pharmaceutical preparations.
General Description
Odorless colorless crystals or white powder. Sublimes readily at 217°F and 54 mm Hg. Cooling saline taste.
Air & Water Reactions
Water soluble. Aqueous solutions are neutral to litmus .
Reactivity Profile
Urethane is incompatible with alkalis, acids, chloral hydrate, camphor, menthol and thymol. Also incompatible with antipyrine and salol. May react with strong oxidizing agents. Liquefies with benzoic acid, resorcinol and salicylic acid. Reacts with phosphorus pentachloride to form an explosive product .
Hazard
Toxic by ingestion.
Safety Profile
Confirmed carcinogen with experimental carcinogenic, neoplastigenic, and tumorigenic data. A transplacental carcinogen. Moderately toxic by ingestion, intraperitoneal, subcutaneous, intramuscular, parenteral, and intravenous routes. An experimental teratogen. Experimental reproductive effects. Human mutation data reported. Causes depression of bone marrow and occasionally focal degeneration in the brain. Can also produce central nervous system depression, nausea and vomiting. Has been found in over 1000 beverages sold in the United States. The most heavily contaminated liquors are bourbons, sherries, and fruit brandies (some had 1000 to 12,000 ppb urethane). Many whiskeys, table and dessert wines, brandies, and liqueurs contain potentially hazardous amounts of urethane. The allowable limit for urethane in alcoholic beverages is 125 ppb. It is formed as a side product during processing.Hot aqueous acids or alkalies decompose urethane to ethanol, carbon dioxide, and ammonia. Reacts with phosphorus pentachloride to form an explosive product. When heated it emits toxic fumes of NOx. Used as an intermedate in the manufacture of pharmaceuticals, pesticides, and fungicides. See also CARBAMATES.
Potential Exposure
Urethane is used as a chemical intermediate in manufacture of pharmaceuticals; pesticides, and fungicides; in the preparation of amino resins. It may be reacted with formaldehyde to give cross-linking agents which impart wash-and-wear properties to fabrics. It has also been used as a solubilizer and cosolvent in the manufacture of pesticides, fumigants, and cosmetics. It was formerly used in the treatment of leukemia. It occurs when diethylpyrocarbonate, a preservative used in wines, fruit juices, and soft drinks, is added to aqueous solutions.
First aid
Skin Contact: Flood all areas of body thathave contacted the substance with water. Do not wait toremove contaminated clothing; do it under the water stream.Use soap to help assure removal. Isolate contaminatedclothing when removed to prevent contact by others.Eye Contact: Remove any contact lenses at once.Immediately flush eyes well with copious quantities ofwater or normal saline for at least 20- 30 min. Seek medicalattention.Inhalation: Leave contaminated area immediately; breathefresh air. Proper respiratory protection must be supplied toany rescuers. If coughing, difficult breathing, or any othersymptoms develop, seek medical attention at once, even ifsymptoms develop many hours after exposure.Ingestion: Contact a physician, hospital, or poison center atonce. If the victim is unconscious or convulsing, do notinduce vomiting or give anything by mouth. Assure thatthis airway is open and lay him on his side with his headlower than his body and transport immediately to a medicalfacility. If conscious and not convulsing, give a glass ofwater or milk to dilute the substance. Vomiting should notbe induced without a physician’s advice.
Carcinogenicity
Urethane is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals.
Environmental Fate
Urethane may be released to the environment in various waste streams. If released to the atmosphere, urethane is expected to exist solely as a vapor in the ambient atmosphere. Vapor-phase urethane is degraded in the atmosphere by reaction with photochemically produced hydroxyl radicals with an estimated half-life of 2.2 days. If released to soil, urethane has very high mobility. Volatilization from moist soil surfaces does not occur. Biodegradation of urethane in soil may be important. If released into water, urethane is not adsorbed to suspended solids and sediment in the water column. Volatilization from water surfaces does not occur. The potential for bioconcentration in aquatic organisms is low based on an estimated bioconcentration factor (BCF) of 0.45. Urethane is resistant to hydrolysis under environmental conditions; hydrolysis half-lives of 3300 and 330 years at pH 7 and 8, respectively, were estimated for urethane. Urethane was judged easy to biodegrade in river die-away tests. Other biodegradation studies using activated sludge indicate urethane may biodegrade slowly.
Metabolism
Not Available
Storage
Color Code—Blue: Health Hazard/Poison: Store ina secure poison location. Prior to working with this chemicalyou should be trained on its proper handling and storage.Urethane must be stored to avoid contact with strong oxidizers (such as chlorine, bromine, and fluorine), strong acids(such as hydrochloric, sulfuric, and nitric), strong bases,camphor, menthol, salol, or thymol since violent reactionsoccur. Store in tightly closed containers in a cool, wellventilated area. A regulated, marked area should be established where this chemical is handled, used, or stored incompliance with OSHA Standard 1910.1045.
Shipping
Toxic solids, organic, n.o.s. require a label of“POISONOUS/TOXIC MATERIALS.” Urethane falls inHazard Class 6.1.
Purification Methods
Urethane is best purified by fractional distillation, but it can be 20 25 1.4144. sublimed at ~103o/~50mm. It has also been recrystallised from *benzene. Its solubilitiy at room temperature is 2g/mL in H2O, 1.25g/mL in EtOH, 1.1g/mL in CHCl3, 0.67g/mL in Et2O and 0.03g/mL in olive oil. It is a suspected human carcinogen.[Beilstein 3 H 22, 3 IV 40.]
Toxicity evaluation
Urethane is activated in the liver into a carcinogenic metabolite. The activation of urethane by cytochrome P450 involves two sequential reactions. First, urethane is dehydrogenated to vinyl carbamate followed by epoxidation to form vinyl carbamate epoxide. The former is believed to be the ultimate carcinogenic metabolite of urethane.
Incompatibilities
Dust may form explosive mixture with air. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, gallium, perchlorate.
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform to EPA regulations governing storage, transportation, treatment, and waste disposal. Controlled incineration (incinerator equipped with a scrubber or thermal unit to reduce nitrogen oxides emissions).
Properties of Urethane
| Melting point: | 48-50 °C(lit.) |
| Boiling point: | 182-184 °C(lit.) |
| Density | 1.10 |
| vapor density | 3.07 (vs air) |
| vapor pressure | 10 mm Hg ( 77.8 °C) |
| refractive index | 1.4144 |
| Flash point: | 198 °F |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | slightly soluble |
| form | Crystals or Crystalline Powder |
| pka | 13.58±0.50(Predicted) |
| color | White |
| PH | pH(50g/l, 25℃) : 5.0~7.0 |
| Water Solubility | slightly soluble |
| Merck | 14,9874 |
| BRN | 635810 |
| Dielectric constant | 14.2(50℃) |
| Stability: | Stable. Incompatible with strong acids, strong bases, strong oxidizing agents. |
| CAS DataBase Reference | 51-79-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Urethane(51-79-6) |
| IARC | 2A (Vol. 7, Sup 7, 96) 2010 |
| EPA Substance Registry System | Urethane (51-79-6) |
Safety information for Urethane
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H350:Carcinogenicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Urethane
Urethane manufacturer
NSR Amino Organics
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 51-79-6 Ethyl carbamate 99%View Details
51-79-6 Ethyl carbamate 99%View Details
51-79-6 -
 Ethyl carbamate CAS 51-79-6View Details
Ethyl carbamate CAS 51-79-6View Details
51-79-6 -
 51-79-6 99%View Details
51-79-6 99%View Details
51-79-6 -
 Ethyl carbamate, ≥99% CAS 51-79-6View Details
Ethyl carbamate, ≥99% CAS 51-79-6View Details
51-79-6 -
 Ethyl Carbamate CAS 51-79-6View Details
Ethyl Carbamate CAS 51-79-6View Details
51-79-6 -
 Ethyl carbamate 98% CAS 51-79-6View Details
Ethyl carbamate 98% CAS 51-79-6View Details
51-79-6 -
 Urethane CAS 51-79-6View Details
Urethane CAS 51-79-6View Details
51-79-6 -
 Powder ETHYL CARBAMATE CHEMICALView Details
Powder ETHYL CARBAMATE CHEMICALView Details
51-79-6
