4-Aminobenzoic acid
Synonym(s):4-Aminobenzoic acid;PABA;para-Aminobenzoic acid;Vitamin Bx;Vitamin H1
- CAS NO.:150-13-0
- Empirical Formula: C7H7NO2
- Molecular Weight: 137.14
- MDL number: MFCD00007894
- EINECS: 205-753-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-25 11:31:46
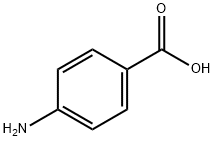
What is 4-Aminobenzoic acid?
Description
4-Aminobenzoic acid (also known as para-aminobenzoic acid or PABA because the number 4 carbon in the benzene ring is also known as the para position) is an organic compound with the formula H2NC6H4CO2H. PABA is a white solid, although commercial samples can appear gray. It is slightly soluble in water. It consists of a benzene ring substituted with amino and a carboxyl groups. The compound occurs naturally. The potassium salt is used as a drug against fibrotic skin disorders, such as Peyronie's disease, under the trade name Potaba. PABA is also occasionally used in pill form by sufferers of irritable bowel syndrome to treat its associated gastrointestinal symptoms, and in nutritional epidemiological studies to assess the completeness of 24-hour urine collection for the determination of urinary sodium, potassium, or nitrogen levels.
Chemical properties
colorless needle-like crystals. turns light yellow in the air or in light. Soluble in hot water, ether, ethyl acetate, ethanol and glacial acetic acid, insoluble in water, benzene, and insoluble in petroleum ether.
Originator
Pabalate,Robins,US,1949
The Uses of 4-Aminobenzoic acid
4-aminobenzoic acid is an aminobenzoic acid isomer that combines with pteridine and glutamic acid to folic acid. The fact that 4-aminobenzoic acid absorbs light throughout the UVB range has also resulted in its use as an ingredient in sunscreens. Also it is used as a component of some medicines e.g analgesic or anesthetic preparations sunscreen agents and bentiromide.
Background
A member of the vitamin B complex. It used to be common in sunscreening agents until found to also be a sensitizer. The potassium salt is used therapeutically in fibrotic skin disorders.
Definition
ChEBI: 4-aminobenzoic acid is an aminobenzoic acid in which the amino group is para to the carboxy group. It has a role as an Escherichia coli metabolite, a plant metabolite and an allergen. It derives from a benzoic acid. It is a conjugate acid of a 4-aminobenzoate.
Preparation
4-Aminobenzoic acid synthesis method: add 38.0g of p-nitrobenzoic acid, 200mL of water, 20mL of tetrahydrofuran, 0.4g of sodium dodecyl sulfonate and 1.9g of Raney nickel to the autoclave, nitrogen replacement Three times, fill with hydrogen, adjust the pressure to 0.9±0.1MPa, adjust the temperature to 100±2°C, and keep the reaction under pressure for 4h to complete. After the reaction, the catalyst was recovered by filtration, left to stand for stratification, the water layer was directly applied mechanically, the tetrahydrofuran layer was distilled and recovered and applied mechanically, then 1.5 g of activated carbon was added to the 4-aminobenzoic acid mother liquor, and under nitrogen protection, heating and refluxing for decolorization for 20min, Filtration, cooling and crystallization of mother liquor, filtration, drying under vacuum at 80-85 °C to obtain 30.3 g of 4-aminobenzoic acid with a yield of 97.2%, a melting point of 187.1-187.6 °C, and a content of 100.2% (permanent stop titration method) .
brand name
RVPaba Lipstick (ICN).
Therapeutic Function
Sunscreen agent, Antirickettsial
Biological Functions
4-Aminobenzoic acid is one of the most important aromatic amino acids. It is an important part of the substances necessary for the growth and division of body cells. It has an irreplaceable role in the metabolism of life. It is used in yeast, liver, bran and malt. The content is very high. 4-Aminobenzoic acid can relieve anemia caused by lack of red blood cells, viral anemia, sprue and anemia during pregnancy. 4-Aminobenzoic acid is a high-efficiency nutritional product with the main ingredient - vitamin B-100, which can effectively improve the three major metabolisms of the human body, comprehensively combat fatigue and relieve stress. The compatibility of 4-aminobenzoic acid with penicillin or streptomycin can effectively improve the bacteriostatic effect.
Synthesis Reference(s)
Synthesis, p. 285, 1971
Tetrahedron, 47, p. 8587, 1991 DOI: 10.1016/S0040-4020(01)82402-9
General Description
Colorless crystals that discolor on exposure to light and air.
Reactivity Profile
4-Aminobenzoic acid is incompatible with ferric salts and oxidizing agents.
Hazard
Questionable carcinogen.
Flammability and Explosibility
Non flammable
Safety Profile
Moderately toxic by ingestion and intravenous routes. Ingesting large doses can cause nausea, vomiting, skin rash, methemoglobinemia, and possibly toxic hepatitis. Experimental reproductive effects. Mutation data reported. Questionable carcinogen. Combustible. When heated to decomposition it emits toxic fumes of NO,. A topical sunscreen.
Purification Methods
Purify p-aminobenzoic acid by dissolving it in 4-5% aqueous HCl at 50-60o, decolorising with charcoal and carefully precipitating it with 30% Na2CO3 to pH 3.5-4 in the presence of ascorbic acid. It can be recrystallised from water, EtOH or EtOH/water mixtures. [Beilstein 14 IV 1126.]
Properties of 4-Aminobenzoic acid
| Melting point: | 187-189 °C(lit.) |
| Boiling point: | 251.96°C (rough estimate) |
| Density | 1.374 g/mL at 25 °C(lit.) |
| vapor pressure | 0Pa at 25℃ |
| refractive index | 1.5323 (estimate) |
| Flash point: | 250 °C |
| storage temp. | 2-8°C |
| solubility | 95% ethanol: soluble5%, clear to slightly hazy, colorless to yellow |
| form | Crystalline Powder |
| pka | 2.50, 4.87(at 25℃) |
| color | White to light yellow |
| Odor | Odorless |
| PH Range | 3.5 |
| PH | 3.5 (5g/l, H2O, 20℃) |
| Water Solubility | 4.7 g/L (20 ºC) |
| Sensitive | Air & Light Sensitive |
| Merck | 14,423 |
| BRN | 471605 |
| Stability: | Stable. Incompatible with strong oxidizing agents. Combustible. Sensitive to light and air. May discolour on exposure to light. |
| CAS DataBase Reference | 150-13-0(CAS DataBase Reference) |
| NIST Chemistry Reference | p-Aminobenzoic acid(150-13-0) |
| IARC | 3 (Vol. 16, Sup 7) 1987 |
| EPA Substance Registry System | p-Aminobenzoic acid (150-13-0) |
Safety information for 4-Aminobenzoic acid
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P501:Dispose of contents/container to..… |
Computed Descriptors for 4-Aminobenzoic acid
| InChIKey | ALYNCZNDIQEVRV-UHFFFAOYSA-N |
4-Aminobenzoic acid manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran



You may like
-
 150- 13-0 98%View Details
150- 13-0 98%View Details
150- 13-0 -
 Para Amino Benzoic Acid 98%View Details
Para Amino Benzoic Acid 98%View Details -
 Para Amino Benzoic Acid PABAView Details
Para Amino Benzoic Acid PABAView Details
150-13-0 -
 p-Aminobenzoic Acid (CAS Number: 150-13-0)View Details
p-Aminobenzoic Acid (CAS Number: 150-13-0)View Details
150-13-0 -
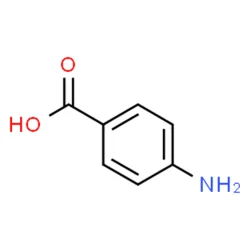 4 Aminobenzoic AcidView Details
4 Aminobenzoic AcidView Details
150-13-0 -
 Para Aminobenzoic AcidView Details
Para Aminobenzoic AcidView Details
150-13-0 -
 Para Amino Benzoic AcidView Details
Para Amino Benzoic AcidView Details
150-13-0 -
 Para Amino Benzoic AcidView Details
Para Amino Benzoic AcidView Details
150-13-0
