Cadmium selenide
- CAS NO.:1306-24-7
- Empirical Formula: CdSe
- Molecular Weight: 191.37
- MDL number: MFCD00010917
- EINECS: 215-148-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-08-19 14:26:28
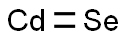
What is Cadmium selenide?
Description
Cadmium selenide (CdSe) is a binary, primarily ionic compound with a touch of covalent character. It can take three crystalline forms, but the hexagonal wurtzite structure shown is by far the most common. The unit cell is shown in the image; the gray balls are cadmium, and the yellow balls are selenium.
In 1879, French chemist M. J. Margottet prepared CdSe by heating elemental cadmium in a current of hydrogen selenide (H2Se), then subliming the product in a hydrogen atmosphere. Since then it also has been produced by treating cadmium chloride or sulfate with H2Se. Now, in the age of nanotechnology, CdSe is made by the reaction of cadmium oxide and elemental selenium in a trialkylphosphine solvent.
CdSe is an n-type semiconductor, which makes it ideal for nanotech applications. Its nanoparticles are especially useful as a component of photocatalysts. In 2016, Philip Kalisman, Yifat Nakibli, and Lilac Amirav* at the Technion–Israel Institute of Technology (Haifa) showed that water splitting via visible-light irradiation with platinum-tipped CdSe@CdS nanorods,?converts 100% of the radiated photons to hydrogen. The authors “believe that this approach . . . will enable us to tackle the remaining challenges with an eye toward overall water splitting.”
Then this year, Wenfa Xie, Hanzhuang Zhang, and colleagues at Jilin University (Changchun, China) used a spray process to make CdSe-containing quantum-dot light-emitting diodes (QLEDs) These QLEDs are?5.4 times more efficient than previously reported similar devices. The authors believe that their results “will spur the progress toward realization of high performance and mass production of all-inorganic QLEDs as a platform for QD-based full-color displays.”
We present CdSe to commemorate?National Nanotechnology Day, October 9th.
Chemical properties
3–12mm pieces (sintered), and -325 mesh 10μm or less with 99.999% purity; white to brown; cub or hexagonal crystal(s); hex: a=0.4309nm, c=0.7021 nm, enthalpy of fusion 305.307 kJ/mol; becomes red in sunlight; used as a red pigment, in semiconductors, and as an evaporation material and sputtering target to produce photoconductive films and infrared filters; possible use in the electrofabrication of microdiode arrays [HAW93] [MER06] [CER91] [KLE93] [KRE91]
Physical properties
The energy gap of the hexagonal CdSe obtained from the absorption measurement Eg (Γ6–Γ1 ) is Eg=1.84 eV, temperature coefficient -4.6×10-4 eV/K, and pressure coefficient 3.7×10-6 eV/kg–cm -2 .
The band structure of cubic CdSe is similar to that of GaAs.
The Uses of Cadmium selenide
Cadmium selenide is used as a n-type semiconductor and its nanoparticles are used in laser diodes, opto-electronic devices, nanosensing, biomedical imaging and high efficiency solar cells. Since, it is transparent to infrared light, it finds application in photoresistors and windows for instruments utilizing IR light.
The Uses of Cadmium selenide
In photoconductors, semiconductors, photoelectric cells, and rectifiers; in phosphors.
The Uses of Cadmium selenide
Dispersions with CdS (Aldrich products 208183, 217921) deposited on glass or Si substrates results in tunable red, yellow, or green fluorescent material.
Definition
ChEBI: Cadmium selenide is a cadmium molecular entity.
Production Methods
A single crystal can be grown artificially through the vapor phase method or the Stockbarger method.
To obtain evaporated films, the source materials are heated in the alumina crucible with a nichrome heater. Crystal with a larger grain size is obtained at a higher substrate temperature.
The following corrosives are reported:
HNO3 (water cleansing after 2–3 s)
H2SO4 (water cleansing after 5–10 s)
18 N solution of 30 HNO3:0.1 HCl:20 H2SO4 (40℃, 8 s, cleansing with concentrated H2SO4)
3 HCl:1 HNO3
What are the applications of Application
Cadmium selenide is used as a photoconductive detector for λ: 0.3–0.72 mm (a sharp peak at 0.7 mm) at room temperature. The typical performances are as follows:
Peak detectivity: 2.1×1011 cm Hz1/2/watt, (λ: 0.7 mm)
Response time: ca. 12 ms
Intermittent frequency: 90 Hz
Dominant noise: current noise
General Description
CdSe crystal can exist in solid hexagonal or cubic structure. CdSe behaves as an n-type semiconductor, with a bandgap of 1.74 eV (300K).1
Flammability and Explosibility
Not classified
Structure and conformation
The space lattice of CdSe (Cadmium selenide) belongs to the two types of crystal system, the cubic system, with the zinc-blend type structure that has a lattice constant of a=0.605 nm, and the hexagonal system, with the wurtzite type structure that has lattice constants of a=0.430 nm and c=0.702 nm.
Properties of Cadmium selenide
| Melting point: | >1350°C |
| Density | 5.81 g/mL at 25 °C(lit.) |
| refractive index | 2.87 (900 nm) |
| storage temp. | 2-8°C |
| solubility | insoluble |
| solubility | insoluble in H2O |
| form | granular |
| appearance | red to black crystals |
| color | Brown-red to black |
| Specific Gravity | 5.81 |
| Water Solubility | Insoluble in water. |
| Sensitive | air sensitive, store cold |
| Crystal Structure | Hexagonal, Wurtzite (Zincite) Structure - Space Group P 63mc |
| Merck | 14,1626 |
| Exposure limits | ACGIH: TWA 0.01 mg/m3; TWA 0.002 mg/m3; TWA 0.2 mg/m3 NIOSH: IDLH 9 mg/m3; IDLH 1 mg/m3; TWA 0.2 mg/m3 |
| Stability: | store cold |
| CAS DataBase Reference | 1306-24-7(CAS DataBase Reference) |
| EPA Substance Registry System | Cadmium selenide (CdSe) (1306-24-7) |
Safety information for Cadmium selenide
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H312:Acute toxicity,dermal H373:Specific target organ toxicity, repeated exposure H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P314:Get medical advice/attention if you feel unwell. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for Cadmium selenide
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran




You may like
-
 Cadmium selenide CAS 1306-24-7View Details
Cadmium selenide CAS 1306-24-7View Details
1306-24-7 -
 Cadmium selenide CAS 1306-24-7View Details
Cadmium selenide CAS 1306-24-7View Details
1306-24-7 -
 Cadmium selenide CAS 1306-24-7View Details
Cadmium selenide CAS 1306-24-7View Details
1306-24-7 -
 Cadmium selenide CAS 1306-24-7View Details
Cadmium selenide CAS 1306-24-7View Details
1306-24-7 -
 Cadmium selenide CAS 1306-24-7View Details
Cadmium selenide CAS 1306-24-7View Details
1306-24-7 -
 Cadmium selenide CAS 1306-24-7View Details
Cadmium selenide CAS 1306-24-7View Details
1306-24-7 -
 Cadmium selenide CASView Details
Cadmium selenide CASView Details -
 Cadmium selenide CAS 1306-24-7View Details
Cadmium selenide CAS 1306-24-7View Details
1306-24-7
