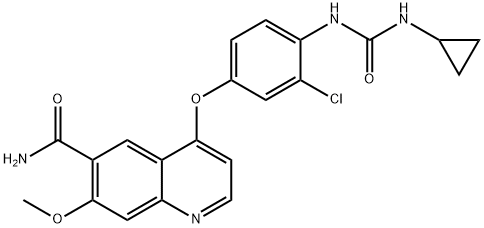
Cabozantinib
- CAS NO.:849217-68-1
- Empirical Formula: C28H24FN3O5
- Molecular Weight: 501.51
- MDL number: MFCD20926324
- EINECS: 692-846-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
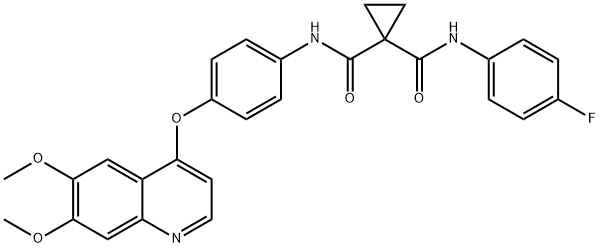
What is Cabozantinib?
Absorption
After oral administration, peak plasma concentration was achieved in 2-5 hours.
Toxicity
Cabozantinib carries a warning of serious gastrointestinal fistulas and perforations, and potentially fatal hemoptysis and gastrointestinal hemorrhage.
Description
Cabozantinib was approved inNovember 2012 for the treatment of patients with progressive, unresectable, locally advanced, or metastatic medullary thyroid cancer (MTC). Cabozantinib was granted orphan drug status by the FDA to facilitate development of newtreatment options for patients with MTC. It is a member of a class of tyrosine kinase inhibitors (TKIs) with nanomolar pan-inhibitory activity against VEGFR2, MET, and RET among others. Inhibition of the VEGF pathway has been shown preclinically to initially slow tumor growth, but rapid revascularization is followed by aggressive tumor growth. The MET pathway has been implicated in the development of VEGF resistance, so dual VEGF/MET activity is viewed as desirable. In addition, mutations in RET play a particular role in MTC, with 25% of the tumors inheriting a germlinemutation in the proto-oncogene, so multiple tyrosine kinase inhibition may be viewed as particularly beneficial for the treatment of MTC.
Originator
Exelixis (United States)
The Uses of Cabozantinib
Cabozantinib (XL184, BMS-907351) is a potent VEGFR2 inhibitor with IC50 of 0.035 nM and also inhibits c-Met, Ret, Kit, Flt-1/3/4, Tie2, and AXL with IC50 of 1.3 nM, 4 nM, 4.6 nM, 12 nM/11.3 nM/6 nM, 14.3 nM and 7 nM, respectively
The Uses of Cabozantinib
XL184 can be used in biological study. Computational network biological approach based on pathway cross-talk inhibition identified new synergistic drug combinations using raloxifene and cabozantinib for treatment of human breast cancer in xenograft mouse model. Potent c-MET inhibitor.
What are the applications of Application
XL-184 free base is a potent VEGFR2 and Met inhibitor
Background
Cabozantinib was first approved in 2012 and is a non-specific tyrosine kinase inhibitor. It was initially approved in the US under the brand name Cometriq, which is indicated for the treatment of metastatic medullary thyroid cancer. In 2016, a capsule formulation (Cabometyx) was approved for the treatment of advanced renal cell carcinoma, and this same formulation gained additional approval in both the US and Canada in 2019 for the treatment of hepatocellular carcinoma in previously treated patients.
Indications
Cabozantinib is indicated for the treatment of progressive, metastatic medullary thyroid cancer. It is also indicated for the treatment of advanced renal cell carcinoma and for hepatocellular carcinoma in patients previously treated with sorafenib.
Definition
ChEBI: A dicarboxylic acid diamide that is N-phenyl-N'-(4-fluorophenyl)cyclopropane-1,1-dicarboxamide in which the hydrogen at position 4 on the phenyl ring is substituted by a (6,7-dimethoxyquinolin-4-yl)oxy group. A multi-t rosine kinase inhibitor, used (as its malate salt) for the treatment of progressive, metastatic, medullary thyroid cancer.
brand name
Cometriq
General Description
Class:receptor tyrosine kinase; Treatment: MTC; RCC; HCC; Other name: XL-184, BMS-907351; Elimination half-life = 110 h; Protein binding > 99.7%
Pharmacokinetics
Cabozantinib suppresses metastasis, angiogenesis, and oncognesis by inhibiting receptor tyrosine kinases.
Clinical Use
Cabozantinib (PF-06463922; brand name Cabometyx; Exelixis, Alameda, CA) is an oral multikinase inhibitor with CNS penetration. It is FDA approved for use in medullary thyroid cancer and as a second-line agent in advanced renal cell carcinoma. In vitro studies found it to exhibit excellent activity against both the wild-type ROS1 fusion and the G2032R and G2026M mutations at concentrations less than 30?nmol/L—a dose much lower than what is clinically achievable [71, 91]. It has been found to inhibit CD74-ROS1-transformed Ba/F3 cells with more potency than entrectinib, brigatinib, lorlatinib [92], or foretinib [71].
Drug interactions
Potentially hazardous interactions with other drugs
Antibacterials: concentration possibly increased by
clarithromycin and erythromycin; concentration
reduced by rifampicin - avoid.
Antiepileptics: concentration possibly reduced
by carbamazepine, fosphenytoin, phenobarbital,
phenytoin and primidone - avoid.
Antipsychotics: avoid with clozapine - increased risk
of agranulocytosis.
Metabolism
Cabozantinib is metabolized mostly by CYP3A4 and, to a minor extent, by CYP2C9. Both enzyme produce an N-oxide metabolite.
Metabolism
Cabozantinib is metabolised mostly by CYP3A4 and, to a minor extent, by CYP2C9. Both enzymes produce an N-oxide metabolite. Cabozantinib is eliminated mainly by the faeces (54%) and also by the urine (27%).
storage
Store at +4°C
References
1) Yakes?et al.?(2011),?Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth; Mol. Cancer Ther.,?10?2298 2) You?et al.?(2011),?VEGF and c-Met blockade amplify angiogenesis inhibition in pancreatic islet cancer; Cancer Res.,?71?4758 3) Kurzrock?et al.?(2011),?Activity of XL184 (Cabozantinib), an oral tyrosine kinase inhibitor in patients with medullary thyroid cancer; J. Clin. Oncol.,?29?2660
Properties of Cabozantinib
| Melting point: | 212-215°C |
| Boiling point: | 758.1±60.0 °C(Predicted) |
| Density | 1.396 |
| storage temp. | -20°C |
| solubility | Soluble in DMSO |
| form | White powder. |
| pka | 13.86±0.70(Predicted) |
| color | White |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 1 month. |
Safety information for Cabozantinib
Computed Descriptors for Cabozantinib
| InChIKey | ONIQOQHATWINJY-UHFFFAOYSA-N |
| SMILES | C1(C(NC2=CC=C(OC3C4C(N=CC=3)=CC(OC)=C(OC)C=4)C=C2)=O)(C(NC2=CC=C(F)C=C2)=O)CC1 |
Cabozantinib manufacturer
Vijaya Pharma And Life Science
Related products of tetrahydrofuran
You may like
-
 849217-68-1 Cabozantinib 98%View Details
849217-68-1 Cabozantinib 98%View Details
849217-68-1 -
 849217-68-1 98%View Details
849217-68-1 98%View Details
849217-68-1 -
 Cabozantinib 849217-68-1 98%View Details
Cabozantinib 849217-68-1 98%View Details
849217-68-1 -
 Cabozantinib >95% CAS 849217-68-1View Details
Cabozantinib >95% CAS 849217-68-1View Details
849217-68-1 -
 Cabozantinib 98.00% CAS 849217-68-1View Details
Cabozantinib 98.00% CAS 849217-68-1View Details
849217-68-1 -
 37951-47-6 3'-Benzyloxy propiophenone, 98% 99%View Details
37951-47-6 3'-Benzyloxy propiophenone, 98% 99%View Details
37951-47-6 -
 104944-18-5 99%View Details
104944-18-5 99%View Details
104944-18-5 -
 3'-Methoxypropiophenone, 99% 37951-49-8 99%View Details
3'-Methoxypropiophenone, 99% 37951-49-8 99%View Details
37951-49-8