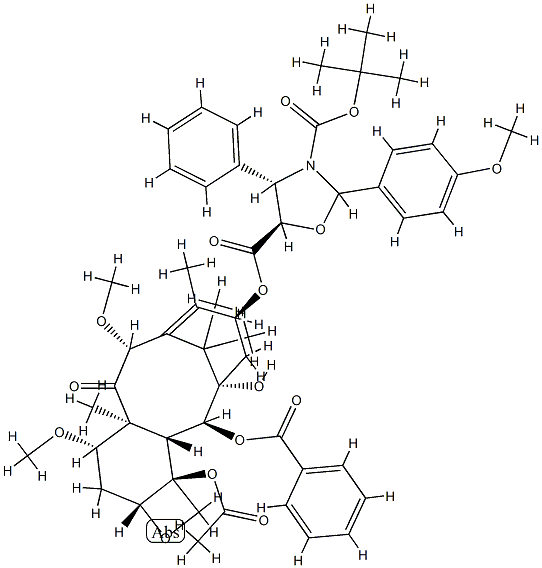
Cabazitaxel
Synonym(s):;DEP cabazitaxel;dimethoxydocetaxel;Jevtana;
- CAS NO.:183133-96-2
- Empirical Formula: C45H57NO14
- Molecular Weight: 835.93
- MDL number: MFCD18827611
- EINECS: 680-632-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
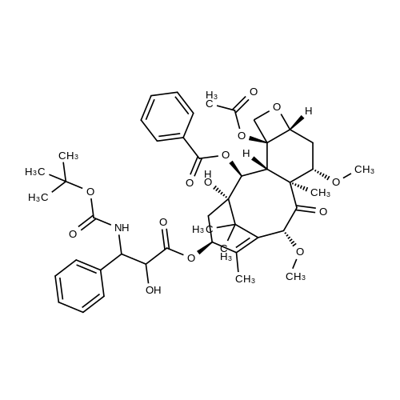
What is Cabazitaxel?
Absorption
Based on the population pharmacokinetic analysis, after an intravenous dose of cabazitaxel 25 mg/m2 every three weeks, the mean Cmax in patients with metastatic prostate cancer was 226 ng/mL (CV 107%) and was reached at the end of the one-hour infusion (Tmax). The mean AUC in patients with metastatic prostate cancer was 991 ng x h/mL (CV 34%). No major deviation from the dose proportionality was observed from 10 to 30 mg/m2 in patients with advanced solid tumours.
Toxicity
The oral LD50 in rats is 500 mg/kg.
Description
In June 2010, the U.S. FDA granted approval for cabazitaxel, known under the names XRP6258 and RPR 116258A, to be used in combination with the steroid prednisone for the treatment of metastatic Castration-Resistant Prostate Cancer (mCRPC), specifically targeting patients who had already been treated with a docetaxel-containing regimen for their late-stage disease. Cabazitaxel is a semisynthetic analog of taxol, a natural product derived from the yew tree's bark, and functions as a microtubule inhibitor by binding to the taxol-binding site on tubulin. This action prevents microtubule disassembly, leading to mitotic blockade and ultimately cell death, a mechanism shared with other taxol class tubulin inhibitors. Despite docetaxel, another semisynthetic taxol analog, being approved for mCRPC treatment in 2004, its role as a substrate for P-glycoprotein (P-gp) is implicated in the development of resistance to taxanes in cancer cells. Cabazitaxel, with its low affinity for P-gp, has demonstrated antitumor activity in both in vitro and in vivo studies, particularly in models where P-gp is overexpressed. It is synthesized commercially from 10-deacetylbaccatin, offering a new therapeutic option for mCRPC patients who have exhausted other treatment options.
Chemical properties
White solid
Originator
Sanofi-Aventis (France)
The Uses of Cabazitaxel
Cabazitaxel (Jevtana, XRP6258) is a novel semi-synthetic taxane with antitumor activity used for the treatment of castration-resistant prostate cancer. A microtubule inhibitor.
Indications
Cabazitaxel is indicated, in combination with prednisone, for the treatment of patients with metastatic castration-resistant prostate cancer previously treated with a docetaxel-containing treatment regimen. In Europe and Canada, it can also be used in combination with prednisolone.
Background
Cabazitaxel is a taxoid synthesized from 10-deacetylbaccatin III, a compound isolated from the yew tree. As a second-generation semisynthetic microtubule inhibitor, cabazitaxel stabilizes microtubules and induces tumour cell death. Due to its low affinity for the P-glycoprotein (P-gp) efflux pump, cabazitaxel can more readily penetrate the blood–brain barrier compared to other taxanes like paclitaxel and docetaxel.
Cabazitaxel is used to treat metastatic castration-resistant prostate cancer. It was first approved by the FDA on June 17, 2010. It was also approved by the EMA on March 17, 2011 and Health Canada on December 17, 2019.
Definition
ChEBI: A tetracyclic diterpenoid that is 10-deacetylbaccatin III having O-methyl groups attached at positions 7 and 10 as well as an O-(2R,3S)-3-[(tert-butoxycarbonyl)amino]-2-hy roxy-3-phenylpropanoyl group attached at position 13. Acts as a microtubule inhibitor, binds tubulin and promotes microtubule assembly and simultaneously inhibits disassembly.
brand name
Jevtana
Clinical Use
Cabazitaxel was developed by Sanofi-Aventis as an intravenous injectable drug for the treatment of hormone-refractory metastatic prostate cancer. As a microtubule inhibitor, cabazitaxel differs from docetaxel because it exhibits a much weaker affinity for P-glycoprotein (P-gp), an adenosine triphosphate (ATP)-dependent drug efflux pump. Cancer cells that express P-gp become resistant to taxanes, and the effectiveness of docetaxel can be limited by its high substrate affinity for P-gp. Clinical studies confirmed that cabazitaxel retains activity in docetaxel-resistant tumors. Common adverse events with cabazitaxel include diarrhea and neutropenia. Cabazitaxel in combination with prednisone is an important new treatment option for men with docetaxel-refractory metastatic CRPC (castration-resistant prostate cancer).
Synthesis
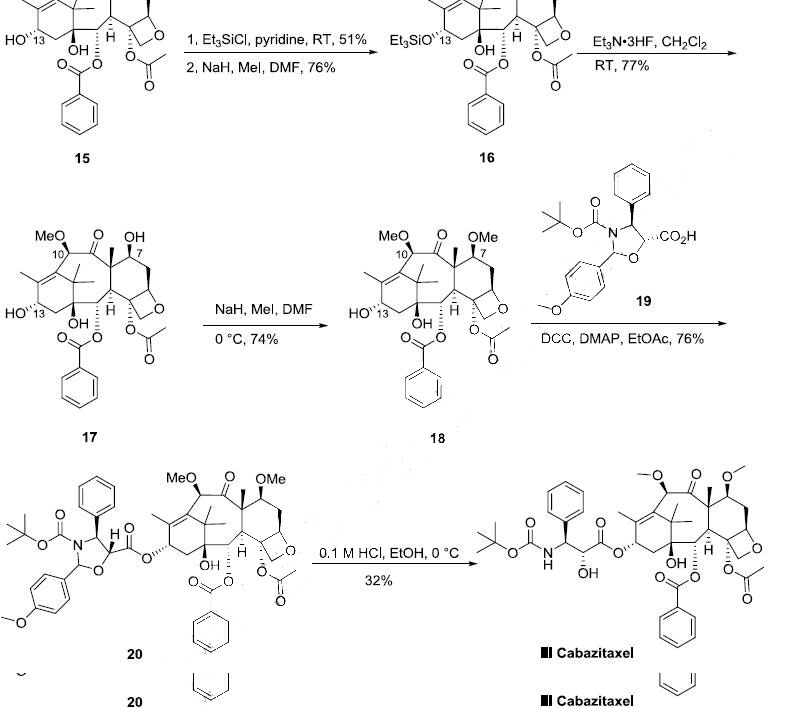
The semi-synthesis of cabazitaxel started from 10- deacetylbaccatin III (15) which can be prepared from 7-xylosyl-10-deacetylbaccatin natural product mixture according to a literature process procedure (the Scheme). 10-Deacetylbaccatin III was protected with triethylsilyl chloride (TESCl) in pyridine to afford the corresponding 7,13-bis-silyl ether in 51% yield, which was methylated with MeI and NaH in DMF to give 10-methoxy-7,13-bis silyl ether 16 in 76% yield. After de-silylation of 16 with triethylamine trihydrofluoride complex at room temperature, triol 17 was obtained in 77% yield. Selective methylation of 17 with MeI and NaH in DMF at 0 oC provided 7,10-dimethyl ether 18 in 74% yield. Compound 18 was condensed with commercially available oxazolidinecarboxylic acid 19 in the presence of dicyclohexylcarbodiimide/dimethylaminopyridine (DCC/DMAP) in ethyl acetate at room temperature to generate ester 20 in 76% yield. The oxazolidine moiety of compound 20 was selectively hydrolyzed under mild acidic conditions to yield the hydroxy Boc-amino ester derivative cabazitaxel (III) in 32% yield.
Drug interactions
Potentially hazardous interactions with other drugs
Antibacterials: Avoid with clarithromycin, rifabutin,
rifampicin and telithromycin.
Antidepressants: Avoid with St John's wort.
Antiepileptics: Avoid with carbamazepine,
fosphenytoin, phenobarbital, phenytoin and
primidone.
Antifungals: Avoid with itraconazole, ketoconazole
and voriconazole.
Antipsychotics: Avoid with clozapine (increased risk
of agranulocytosis).
Antivirals: Avoid with atazanavir, indinavir, ritonavir
and saquinavir.
Biologiacal activity
Cabazitaxel demonstrates a broad spectrum of antitumour activity against advanced human tumours xenografted in mice, including intracranial human glioblastomas. Cabazitaxel has a low affinity to P-glycoprotein, allowing it to penetrate the blood-brain barrier without being subject to extensive P-gp-mediated active efflux. Cabazitaxel works against docetaxel-sensitive tumours and tumour models resistant to docetaxel and other chemotherapy drugs.
Metabolism
More than 95% of cabazitaxel is extensively metabolized in the liver. CYP3A4 and CYP3A5 are responsible for 80% to 90% of drug metabolism, while CYP2C8 is involved to a lesser extent. While cabazitaxel is the main circulating moiety in human plasma, seven metabolites have been detected in plasma, including three active metabolites arising from O-demethylation - docetaxel, RPR112698, and RPR123142. The main metabolite accounts for 5% of total cabazitaxel exposure.
Properties of Cabazitaxel
| Melting point: | 180 °C |
| Boiling point: | 870.7±65.0 °C(Predicted) |
| Density | 1.31 |
| storage temp. | Inert atmosphere,Store in freezer, under -20°C |
| solubility | Chloroform (Slightly), DMSO (Slightly), Methanol (Slightly) |
| form | White solid. |
| pka | 11.20±0.46(Predicted) |
| color | White to Off-White |
Safety information for Cabazitaxel
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H341:Germ cell mutagenicity H373:Specific target organ toxicity, repeated exposure |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Cabazitaxel
Cabazitaxel manufacturer
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran

You may like
-
 183133-96-2 Cabazitaxel 98%View Details
183133-96-2 Cabazitaxel 98%View Details
183133-96-2 -
 183133-96-2 98%View Details
183133-96-2 98%View Details
183133-96-2 -
 Cabazitaxel 95% CAS 183133-96-2View Details
Cabazitaxel 95% CAS 183133-96-2View Details
183133-96-2 -
 Cabazitaxel CAS 183133-96-2View Details
Cabazitaxel CAS 183133-96-2View Details
183133-96-2 -
 Cabazitaxel 183133-96-2 / 1402820-62-5 98%View Details
Cabazitaxel 183133-96-2 / 1402820-62-5 98%View Details
183133-96-2 / 1402820-62-5 -
 Cabazitaxel 98%View Details
Cabazitaxel 98%View Details
183133-96-2 / 1402820-62-5 -
 Cabazitaxel 98% (HPLC) CAS 183133-96-2View Details
Cabazitaxel 98% (HPLC) CAS 183133-96-2View Details
183133-96-2 -
 Cabazitaxel CAS 183133-96-2View Details
Cabazitaxel CAS 183133-96-2View Details
183133-96-2
