Ceritinib (LDK378)
- CAS NO.:1032900-25-6
- Empirical Formula: C28H36ClN5O3S
- Molecular Weight: 558.14
- MDL number: MFCD26142648
- EINECS: 811-457-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 21:30:31
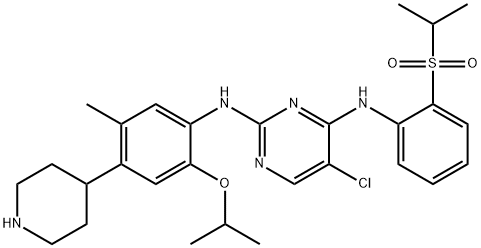
What is Ceritinib (LDK378)?
Absorption
After oral administration of ceritinib, peak concentrations were achieved after approximately 4 to 6 hours.
Toxicity
There is not currently any data on carcinogenicity, effect on human fertility, or on early embryonic development. However, based on its mechanism of action, ceritinib may cause fetal harm when administered to pregnant women and should therefore be administered with effective contraception during treatment. Diarrhea, nausea, vomiting, or abdominal pain occurred in 96% of 255 patients including severe cases in 14% of patients. Drug-induced hepatotoxicity also occurred in 27% of 255 patients, presenting as alanine aminotransferase (ALT) levels greater than 5 times the upper limit of normal (ULN). Severe, life-threatening, or fatal interstitial lung disease (ILD)/pneumonitis, hyperglycaemia, and bradycardia have also been reported.
Description
Ceritinib (previously LDK378l, brand name Zykadia; Novartis Pharmaceuticals) is an oral small molecule tyrosine kinase inhibitor of ALK [87]. Preclinical studies suggested that it would inhibit ROS1 as well [88, 89].
The Uses of Ceritinib (LDK378)
5-Chloro-N2-(2-isopropoxy-5-methyl-4-(piperidin-4-yl)phenyl)-N4-(2-(isopropylsulfonyl)phenyl)pyrimidine-2,4-diamine, is a Anaplastic lymphoma kinase (ALK) inhibitor.
Indications
Ceritinib is a kinase inhibitor indicated for the treatment of patients with anaplastic lymphoma kinase (ALK)-positive metastatic non-small cell lung cancer (NSCLC) who have progressed on or are intolerant to crizotinib. This indication is approved under accelerated approval based on tumor response rate and duration of response. An improvement in survival or disease-related symptoms has not been established. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.
Background
Ceritinib is used for the treatment of adults with anaplastic lymphoma kinase (ALK)-positive metastatic non-small cell lung cancer (NSCLC) following failure (secondary to resistance or intolerance) of prior crizotinib therapy. About 4% of patients with NSCLC have a chromosomal rearrangement that generates a fusion gene between EML4 (echinoderm microtubule-associated protein-like 4) and ALK (anaplastic lymphoma kinase), which results in constitutive kinase activity that contributes to carcinogenesis and seems to drive the malignant phenotype. Ceritinib exerts its therapeutic effect by inhibiting autophosphorylation of ALK, ALK-mediated phosphorylation of the downstream signaling protein STAT3, and proliferation of ALK-dependent cancer cells. Following treatment with crizotinib (a first-generation ALK inhibitor), most tumours develop drug resistance due to mutations in key "gatekeeper" residues of the enzyme. This occurrence led to development of novel second-generation ALK inhibitors such as ceritinib to overcome crizotinib resistance. The FDA approved ceritinib in April 2014 due to a surprisingly high response rate (56%) towards crizotinib-resistant tumours and has designated it with orphan drug status.
Definition
ChEBI: A member of the class of aminopyrimidines that is 2,6-diamino-5-chloropyrimidine in which the amino groups at positions 2 and 6 are respectively carrying 2-methoxy-4-(piperidin-4-yl)-5-methylphenyl and 2-(isopropylsulfonyl)phenyl substituents. Used for the treatment of ALK-positive metastatic non-small cell lung cancer.
Indications
Ceritinib (Zykadia(R), Novartis), approved in 2014, was developed as a second-generation ALK inhibitor for patients with NSCLC who have developed crizotinib resistance. Ceritinib addresses two of the most common ALK mutants that lead to crizotinib resistance, L1196M andG1269A, but is ineffective for G1202R and F1174C variants of ALK.
General Description
Class: receptor tyrosine kinase; Treatment: NSCLC; Elimination half-life = 41 h; Protein binding = 97%
Trade name
Zykadia?
Clinical Use
ALK-inhibitor:
Treatment of anaplastic lymphoma kinase (ALK)-
positive advanced non-small cell lung cancer
(NSCLC) previously treated with crizotinib
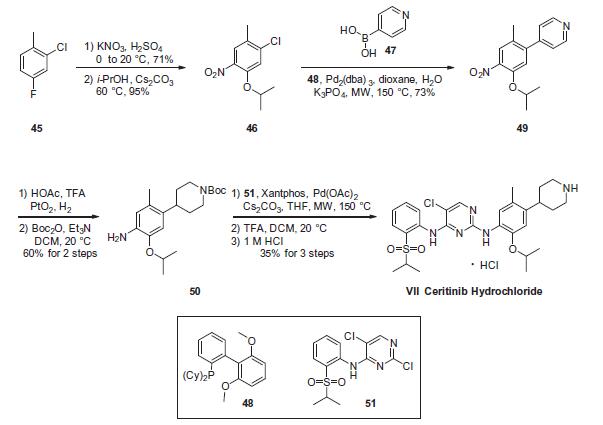
Synthesis
The critical step in the synthesis of ceritinib involves a latestage
Buchwald¨CHartwig coupling of two advanced intermediates,
anilino piperidine 50 and arylsulfonyl chloro-pyrimidine 51. While these conditions utilize microwave-mediated
conditions (as does another Suzuki coupling within the sequence),
which are not commonly employed for process-scale routes, to our
knowledge no other conditions to have been reported to date
which facilitate the construction of ceritinib.
Construction of anilino piperidine 50 commenced with 2-
chloro-4-fluoro-1-methylbenzene (45). Nitration with KNO3/
H2SO4 and subsequent reaction with i-PrOH/Cs2CO3 at elevated
temperatures provided the 5-isopropoxy chloride intermediate
46 in 67% over 2 steps. Suzuki coupling of 46 with 4-pyridine boronic
acid (47) furnished 49 in 73% yield, which was then subjected
to platinum oxide-catalyzed hydrogenation conditions in the presence
of acetic acid and trifluoroacetic acid, affording the corresponding
piperidinyl aniline intermediate. Immediate Bocprotection
of the crude aniline provided the Buchwald¨CHartwig
coupling precursor 50 in 60% over 2 steps. Next, the critical union
of 50 and 51 via Buchwald¨CHartwig coupling furnished the framework
of ceritinib. This was followed by removal of the Boc group
with TFA and subsequent precipitation with 1 M HCl to yield ceritinib
(VII) as the HCl salt in 35% yield from 50.
Drug interactions
Potentially hazardous interactions with other drugs
Anti-arrhythmics: possibly increased risk of
ventricular arrhythmias with amiodarone,
disopyramide, dronedarone and flecainide.
Antibacterials: possibly increased risk of ventricular
arrhythmias with bedaquiline, clarithromycin,
delamanid, IV erythromycin, moxifloxacin and
telavancin; concentration reduced by rifampicin and
possibly rifabutin - avoid.
Antidepressants: risk of QT prolongation with
citalopram, escitalopram, venlafaxine and tricyclics
that prolong the QT interval - avoid; concentration
possibly reduced by St John’s wort - avoid.
Anti-emetics: possibly increased risk of ventricular
arrhythmias with domperidone and ondansetron.
Antiepileptics: possibly increased concentration
with carbamazepine - avoid; concentration possibly
reduced by fosphenytoin, phenobarbital, phenytoin
and primidone.
Antifungals: concentration increased by ketoconazole
and possibly itraconazole, posaconazole and
voriconazole - avoid or reduce ceritinib dose
Antihistamines: avoid with hydralazine due to risk of
QT prolongation.
Antimalarials: possibly increased risk of ventricular
arrhythmias with artemether and lumefantrine,
piperaquine with artenimol, chloroquine and quinine
- avoid.
Antipsychotics: possibly increased risk of ventricular
arrhythmias with droperidol and haloperidol;
avoid with other antipsychotics that prolong the
QT interval; increased risk of agranulocytosis with
clozapine - avoid.
Antivirals: concentration possibly increased by
atazanavir, fosamprenavir, lopinavir, ritonavir,
saquinavir and tipranavir - avoid or reduce dose; risk
of QT prolongation with dasatinib - avoid.
Apomorphine: risk of QT prolongation - avoid.
Beta-blockers: possibly increased risk of ventricular
arrhythmias with sotalol.
Ciclosporin: may increase ciclosporin concentration
- avoid.
Cobicistat: concentration of ceritinib increased -
avoid or adjust ceritinib dose.
Cytotoxics: risk of QT prolongation with arsenic
trioxide, bosutinib, cabozantib, crizotinib, eribulin,
lapatinib, nilotinib, osimertinib, panobinostat,
pazopanib, sorafenib, sunitinib, vandetanib,
vemurafenib, vinflunine - avoid; concentration possibly increased by idelalisib - avoid or adjust
ceritinib dose
Enzalutamide: increases ceritinib concentration -
avoid.
Methadone: possibly increased risk of ventricular
arrhythmias.
Pasireotide: possibly increased risk of ventricular
arrhythmias - avoid.
Ranolazine: possibly increased risk of ventricular
arrhythmias - avoid.
Sirolimus: avoid concomitant use.
Tacrolimus: avoid concomitant use.
Tetrabenazine: possibly increased risk of ventricular
arrhythmias - avoid.
Tizanidine: possibly increased risk of ventricular
arrhythmias - avoid.
Warfarin - avoid concomitant use.
Metabolism
In vitro studies demonstrated that CYP3A was the major enzyme involved in the metabolic clearance of ceritinib. Following oral administration of a single 750 mg radiolabeled ceritinib dose, ceritinib as the parent compound was the main circulating component (82%) in human plasma.
Metabolism
In vitro studies demonstrated that CYP3A was the major enzyme involved in the metabolic clearance of ceritinib. The main route of excretion of ceritinib and its metabolites is via the faeces. Recovery of unchanged ceritinib in the faeces accounts for a mean of 68% of a dose.
Storage
Store at +4°C
References
[1]chen j, jiang c, wang s. ldk378: a promising anaplastic lymphoma kinase (alk) inhibitor. j med chem. 2013 jul 25;56(14):5673-4. doi: 10.1021/jm401005u. epub 2013 jul 9.
[2]marsilje th, pei w, chen b, lu w, uno t, jin y, jiang t, kim s, li n, warmuth m, sarkisova y, sun f, steffy a, pferdekamper ac, li ag, joseph sb, kim y, liu b, tuntland t, cui x, gray ns, steensma r, wan y, jiang j, chopiuk g, li j, gordon wp, richmond w, johnson k, chang j, groessl t, he yq, phimister a, aycinena a, lee cc, bursulaya b, karanewsky ds, seidel hm, harris jl, michellys py. synthesis, structure-activity relationships, and in vivo efficacy of the novel potent and selective anaplastic lymphoma kinase (alk) inhibitor 5-chloro-n2-(2-isopropoxy-5-methyl-4-(piperidin-4-yl)phenyl)-n4-(2-(isopropylsulfonyl)phenyl)pyrimidine-2,4-diamine (ldk378) currently in phase 1 and phase 2
Properties of Ceritinib (LDK378)
| Melting point: | 173-175°C |
| Boiling point: | 720.7±70.0 °C(Predicted) |
| Density | 1.251±0.06 g/cm3(Predicted) |
| storage temp. | -20°C Freezer |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | Off-white solid. |
| pka | 10.16±0.10(Predicted) |
| color | White to Off-White |
Safety information for Ceritinib (LDK378)
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H320:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Ceritinib (LDK378)
Ceritinib (LDK378) manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 1032900-25-6 Ceritinib 98%View Details
1032900-25-6 Ceritinib 98%View Details
1032900-25-6 -
 1032900-25-6 98%View Details
1032900-25-6 98%View Details
1032900-25-6 -
 1032900-25-6 Ceritinib 98%View Details
1032900-25-6 Ceritinib 98%View Details
1032900-25-6 -
 CERITINIB 95-99%View Details
CERITINIB 95-99%View Details
1032900-25-6 -
 Ldk378 95% CAS 1032900-25-6View Details
Ldk378 95% CAS 1032900-25-6View Details
1032900-25-6 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4
