BROMOTRIFLUOROMETHANE
- CAS NO.:75-63-8
- Empirical Formula: CBrF3
- Molecular Weight: 148.91
- MDL number: MFCD00000370
- EINECS: 200-887-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:57
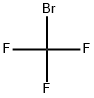
What is BROMOTRIFLUOROMETHANE?
Description
Bromotrifluoromethane (CBrF3) is a colorless, odorless gas that, under the name Halon 1301 and others, primarily was used as a fire suppressant. It and other bromofluorocarbons were synthesized in 1946 by T. J. Brice, W. H. Pearlson, and J. H. Simons at Penn State, then known as Pennsylvania State College. Their general method was to heat hydrogen-containing fluorocarbons with molecular bromine at 40–50 °C.
Halon 1301 and other halocarbons were used instead of water and aqueous solutions to combat fires in locations where valuable items such as computers and museum pieces would ?be harmed by water damage. Its extreme health hazards (see the hazard information table) mandated limited human exposure .
In addition to its use as a fire suppressant, CBrF3 was used as a refrigerant; it is also a precursor to the trifluoromethylating agent trifluoromethyltrimethylsilane, the Molecule of the Week for April 12, 2021. CBrF3, however, is an atmospheric ozone-depleting agent; when the Montreal Protocol was instituted in 1989, it and other Halon fire suppressants and refrigerants had to be replaced.
One CBrF3 replacement is 1,1,1,2,2,4,5,5,5-nonafluoro-4-(trifluoromethyl)pentan-3-one1, which, under the name Novec 1230, is a liquid (bp 49 °C) fire suppressant. As with CBrF3, it is used to avoid damage to items of value. A purer form of this molecule, Novec 649, is marketed as a low-temperature heat-transfer fluid.
1. CAS Reg. No. 756-13-8.
Chemical properties
Trifluorobromomethane is a colorless gas with a slight ethereal odor. Shipped as a liquefied compressed gas.
The Uses of BROMOTRIFLUOROMETHANE
Used as fire extinguishing agent for oil, electrical equipment, organic solvent, natural gas and a variety of organics, especially for important military and civilian sites.
The Uses of BROMOTRIFLUOROMETHANE
Fire extinguishing agent; refrigerant.
Synthesis Reference(s)
Journal of the American Chemical Society, 68, p. 968, 1946 DOI: 10.1021/ja01210a017
General Description
A colorless, odorless gas at room conditions Shipped as a liquid confined under its own vapor pressure. Noncombustible. Nontoxic but can asphyxiate by the displacement of air. Contact with the unconfined liquid can cause frostbite by evaporative cooling. Exposure of the container to prolonged heat or fire can cause BROMOTRIFLUOROMETHANE to rupture violently and rocket.
Air & Water Reactions
Slightly soluble in water.
Reactivity Profile
BROMOTRIFLUOROMETHANE may react with aluminum to produce substantial heat. Other halogenated hydrocarbons, such as fluorotrichloromethane, dichlorodifluoromethane, chlorodifluoromethane, tetrafluoromethane produce sufficient heat in this way to melt aluminum pieces. The vigor of the reaction appears to depend on the degree of fluorination and the vapor pressure [Chem. Eng. News 39(27):44 1961].
Health Hazard
Vapors may cause dizziness or asphyxiation without warning. Vapors from liquefied gas are initially heavier than air and spread along ground. Contact with gas or liquefied gas may cause burns, severe injury and/or frostbite. Fire may produce irritating, corrosive and/or toxic gases.
Fire Hazard
Some may burn but none ignite readily. Containers may explode when heated. Ruptured cylinders may rocket.
Safety Profile
Wildly toxic by inhalation. Incompatible with aluminum. When heated to decomposition it emits toxic fumes of Fand Br-. See also BROMIDES and FLUORIDES.
Potential Exposure
This material is used as a fire extinguishing agent, a chemical intermediate, and as a refrigerant.
Shipping
UN1009 Bromotrifluoromethane or Refrigerant gas, R-13B, Hazard Class: 2.2; Labels: 2.2-Nonflammable gas. Cylinders must be transported in a secure upright position, in a well-ventilated truck. Protect cylinder and labels from physical damage. The owner of the compressed gas cylinder is the only entity allowed by federal law (49CFR) to transport and refill them. It is a violation of transportation regulations to refill compressed gas cylinders without the express written permission of the owner.
Purification Methods
Purify the gas by passing it through a tube containing P2O5 on glass wool into a vacuum system where it is frozen out in a quartz tube and degassed by a cycles of freezing, evacuating and thawing. [Beilstein 1 III 83, 1 IV 73.]
Incompatibilities
Keep away from chemically active metals, such as calcium, powdered aluminum; zinc, magnesium. Attacks some plastics, rubber, and coatings.
Waste Disposal
Return refillable compressed gas cylinders to supplier. Incineration, preferably after mixing with another combustible fuel. Care must be exercised to assure complete combustion to prevent the formation of phosgene. An acid scrubber is necessary to remove the halo acids produced.
Properties of BROMOTRIFLUOROMETHANE
| Melting point: | -168°C |
| Boiling point: | -58°C |
| Density | 1,58 g/cm3 |
| vapor pressure | >760 at 20 °C (NIOSH, 1997) |
| refractive index | 1.2380 |
| solubility | Soluble in chloroform (Weast, 1986) and many other solvents, particularly chlorinated
hydrocarbons. |
| form | A gas |
| appearance | colorless gas |
| color | Colorless gas with an ether-like odor |
| Water Solubility | 0.03 wt % at 20 °C (NIOSH, 1997) |
| Henry's Law Constant | (atm?m3/mol):
0.500 at 25 °C (Hine and Mookerjee, 1975) |
| Exposure limits | NIOSH REL: TWA 1,000 ppm (6,100 mg/m3), IDLH 40,000 ppm; OSHA PEL:
TWA 1,000 ppm. |
| CAS DataBase Reference | 75-63-8(CAS DataBase Reference) |
| EPA Substance Registry System | Halon 1301 (75-63-8) |
Safety information for BROMOTRIFLUOROMETHANE
Computed Descriptors for BROMOTRIFLUOROMETHANE
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran





You may like
-
![Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%View Details
Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%View Details
61397-56-6 -
 287930-77-2 / 142569-70-8 99%View Details
287930-77-2 / 142569-70-8 99%View Details
287930-77-2 / 142569-70-8 -
![2033-24-1 2,2-Dimethyl-1,3-Dioxane-4,6-Dione [Meldrum Acid] 98%](https://img.chemicalbook.in//Content/image/CP5.jpg) 2033-24-1 2,2-Dimethyl-1,3-Dioxane-4,6-Dione [Meldrum Acid] 98%View Details
2033-24-1 2,2-Dimethyl-1,3-Dioxane-4,6-Dione [Meldrum Acid] 98%View Details
2033-24-1 -
 Ethyl-2-Chloroacetoacetate 609-15-4View Details
Ethyl-2-Chloroacetoacetate 609-15-4View Details
609-15-4 -
 CIS- BROMO BENZOATEView Details
CIS- BROMO BENZOATEView Details
61397-56-6 -
 609-15-4View Details
609-15-4View Details
609-15-4 -
![1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) 1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%View Details
1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%View Details
221615-75-4 -
 27143-07-3View Details
27143-07-3View Details
27143-07-3
