Bromazepam
- CAS NO.:1812-30-2
- Empirical Formula: C14H10BrN3O
- Molecular Weight: 316.15
- MDL number: MFCD00057902
- EINECS: 217-322-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-06-08 09:02:13
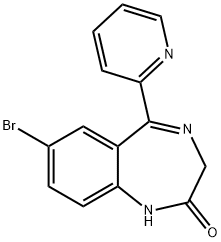
What is Bromazepam?
Absorption
Bioavailability is 84% following oral administration. The time to peak plasma level is 1 - 4 hours. Bromazepam is generally well absorbed after oral administration.
Description
Bromazepam is a psychoactive benzodiazepine derivative and prescription drug that is commonly abused and often used in ‘drug-
Chemical properties
Crystalline Solid
Originator
Lexotan,Roche,Italy,1975
The Uses of Bromazepam
Controlled substance (depressant). Anxiolytic.
Indications
For the short-term treatment of insomnia, short-term treatment of anxiety or panic attacks, if a benzodiazepine is required, and the alleviation of the symptoms of alcohol- and opiate-withdrawal.
Background
One of the benzodiazepines that is used in the treatment of anxiety disorders. It is a Schedule IV drug in the U.S. and Canada and under the Convention on Psychotropic Substances. It is a intermediate-acting benzodiazepine.
Definition
ChEBI: Bromazepam is an organic molecular entity.
Manufacturing Process
Example: 32.8 grams of 2-(2-aminobenzoyl)-pyridine and 200 cc of acetic
anhydride were stirred at room temperature for 3 hours and then permitted to
stand overnight. Evaporation to dryness and digestion of the residue with 200
cc of water containing a little sodium bicarbonate to make the pH slightly
alkaline gave 2-(2-acetamidobenzoyl)-pyridine as a light tan powder, which
upon crystallization from methanol formed colorless crystals melting at 151°-
153°C.
A solution of 8.6 cc of bromine in 100 cc of acetic acid was added slowly over
a 3.5 hour period to a stirred solution of 38.5 grams of 2-(2-
acetamidobenzoyl)-pyridinein 250 cc of acetic acid. The dark solution was
stirred for another 3 hours, permitted to stand over night, stirred for 1 hour
with N2 sweeping, and evaporated at diminished pressure in the hood. The
gummy residue (75 grams) was treated with water and ether, made alkaline
with dilute sodium bicarbonate solution, and separated. Both phases contained
undissolved product which was filtered off. Additional crops were obtained by
further extraction of the aqueous phase with ether and evaporation of the
resulting ether solutions. All these materials were recrystallized from methanol
(decolorizing carbon added) yielding 2-(2-acetamido-5-bromobenzoyl)-
pyridineas yellow crystals melting at 131.5°-133°C.
20.85 grams of 2-(2-acetamido-5-bromobenzoyl)-pyridinein 250 cc of 20%
hydrochloric acid in ethanol were heated to reflux for 2 hours. 100 cc of
alcohol were added after one hour to maintain fluidity. The mixture stood
overnight, was chilled and filtered to give 20.5 grams of colorless crystalline
2-(2-amino-5-bromobenzoyl)-pyridinehydrochloride. Digestion of this hydrochloride with 0.5liter hot water hydrolyzed this product to the free base,
2-(2-amino-5-bromobenzoyl)-pyridine which formed yellow crystals, melting
at 98°-100°C. Evaporation of the alcoholic mother liquor, water digestion of
the residue, and alkalization of the water digests afforded additional crops of
2-(2-amino-5-bromobenzoyl)pyridine.
0.145 kg of 2-(2-amino-5-bromobenzoyl)-pyridine, was dissolved in 2.0 liters
of glacial acetic acid. The resultant solution was placed in a 3 liter, 3-necked,
round bottom flask fitted with a stirrer, thermometer and dropping funnel. The
system was protected by a drying tube filled with anhydrous calcium chloride.
To the solution, with stirring at room temperature, were carefully added 46.7
ml of bromoacetyl bromide. After the addition was completed, the stirring was
continued for two hours. The mixture was then warmed to 40°C, stirred at
that temperature for 1.5 hours, chilled and filtered. The residue, after being
washed with glacial acetic acid, was dried in vacuo over flake potassium
hydroxide to give 2-(2-bromoacetamido-5-bromobenzoyl)-
pyridinehydrobromide orange crystals, MP 205°-206°C, dec.
The hydrobromide was hydrolyzed to the free base as follows: 0.119 kg of 2-
(2-bromoacetamido-5-bromobenzoyl)-pyridine hydrobromide was stirred with
1.2 liters of cold water for 3.5 hours. The mixture was chilled and filtered, and
the residue washed with cold water and dried to give 2-(2-bromoacetamido-5-
bromobenzoyl)-pyridine, MP 101°C (sinters), 103°-106°C, dec.
93.0 grams of 2-(2-bromoacetamido-5-bromobenzoyl)-pyridinewas carefully
added to 0.5 liter of anhydrous ammonia in a 1 liter, 3-necked, round bottom
flask equipped with stirrer and reflux condenser and cooled by a Dry Iceacetone
bath. The system was protected from moisture by a drying tube
containing anhydrous calcium chloride. After stirring for 2 hours, the cooling
bath was removed. The mixture was then stirred for 6 hours, during which
time the ammonia gradually boiled off. 0.4 liter of water was added to the
solid residue and stirrind was resumed for about 2 hours. The solid was then
filtered off, washed with water and dried in vacuo over potassium hydroxide
flakes. The residue was dissolved on a steam bath in 1.4 liters of ethyl
alcohol-acetonitrile (1:1) (decolorizing charcoal added). The solution was
filtered hot and the filtrate chilled overnight. The crystalline deposit was
filtered off, washed with cold ethyl alcohol and dried in vacuo over flake
potassium hydroxide to give 54.2 grams. 7-Bromo-1,3-dihydro-5-(2-pyridyl)-
2H-1,4-benzodiazepin-2-one, MP 238°C (sinters), 239°-240.5°, dec. Further
processing of the mother liquor yielded additional product.
brand name
Lectopam (Roche, Puerto Rico).
Therapeutic Function
Tranquilizer
Pharmacokinetics
Bromazepam is a lipophilic, long-acting benzodiazepine and with sedative, hypnotic, anxiolytic and skeletal muscle relaxant properties. It does not possess any antidepressant qualities. Bromazepam, like other benzodiazepines, presents a risk of abuse, misuse, and dependence. According to many psychiatric experts, Bromazepam has a greater abuse potential than other benzodiazepines because of fast resorption and rapid onset of action.
Enzyme inhibitor
This lipophilic valium-like benzodiazepine (FW = 316.20 g/mol; CAS 1812- 30-2; IUPAC Name: 7-bromo-5-(pyridin-2-yl)-1H-benzo[e][1,4]diazepin- 2(3H)-one), is a long-lasting anxiolytic agent used to treat anxiety or panic states. Like diazepam, bromazepam is a positive allosteric modulator of GABAA receptors, promoting GABA binding, which in turn increases the total conduction of chloride ions across the neuronal cell membrane. Like other benzodiazepines, bromazepam also inhibits the acetylcholine receptoroperated potassium current. Bromazepam is mainly metabolized by oxidative pathways within the liver.
Metabolism
Hepatically, via oxidative pathways (via an enzyme belonging to the Cytochrome P450 family of enzymes). One of the main metabolites is 3-hydroxybromazepam. It is pharmacologically active and the half life is similar to that of the parent compound.
Properties of Bromazepam
| Melting point: | 237-238.5oC (dec.) |
| Boiling point: | 490.7±45.0 °C(Predicted) |
| Density | 1.5573 (rough estimate) |
| refractive index | 1.6520 (estimate) |
| Flash point: | 11 °C |
| storage temp. | 2-8°C |
| solubility | Practically insoluble in water, slightly soluble or sparingly soluble in ethanol (96 per cent) and in methylene chloride. |
| pka | pKa 2.5(spectro,
polarographic) (Uncertain);5.2(spectro,
polarographic) (Uncertain);11.8(spectro,
polarographic) (Uncertain) |
| form | A crystalline solid |
| CAS DataBase Reference | 1812-30-2(CAS DataBase Reference) |
| NIST Chemistry Reference | Bromazepam(1812-30-2) |
Safety information for Bromazepam
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H225:Flammable liquids H370:Specific target organ toxicity, single exposure |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P311:Call a POISON CENTER or doctor/physician. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for Bromazepam
Bromazepam manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1