DBU
Synonym(s):1,8-Diazabicyclo[5.4.0]undec-7-ene;2,3,4,6,7,8,9,10-Octahydropyrimidol[1,2-a]azepine;2,3,4,6,7,8,9,10-Octahydropyrimidol[1,2-a]azepine;DBU
- CAS NO.:6674-22-2
- Empirical Formula: C9H16N2
- Molecular Weight: 152.24
- MDL number: MFCD00006930
- EINECS: 229-713-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:57
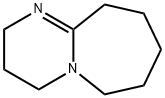
What is DBU?
Description
DBU is a bicyclic amidine base. It is non-nucleophilic, sterically hindered, tertiary amine base in organic chemistry. It is reported to be superior to amine catalyst in Baylis-Hillman reaction.It promotes the methylation reaction of phenols, indoles and benzimidazoles with dimethyl carbonate under mild conditions.
Description
DBU is considered to be a non-nucleophilic base. DBU can serve as a base, catalyst, or complexing ligand in a wide variety of reactions.
Chemical properties
Colorless to yellow liquid
Occurrence
Although all commercially available DBU is produced synthetically, it may also be isolated from the sea sponge Niphates digitalis.The biosynthesis of DBU has been proposed to begin with 1,6-hexanedial and 1,3-diaminopropane.
The Uses of DBU
DBU may be used:
- as catalyst for carboxylic acid esterification with dimethyl carbonate
- in the synthesis of duocarmycin and CC-1065 analogs
- as catalyst in aza-Michael addition and Knovenegal condensation reaction
- as base for dehalogenation of halogenated Diels-Alder adducts and the resulting activated 2,4-dienones were subjected to regio- and stereo-directed Michael additions, using Yamamoto′s reagent (CH3Cu · BF3)
- in a new synthesis of the ABCD ring system of Camptothecin
- DBU may be used as an catalyst for the dissolution and activation of cellulose by a reversible reaction of its hydroxyl groups with carbon dioxide. This dissolved cellulose system can be derivatized to form cellulose mixed esters.
- Used in a new synthesis of the ABCD ring system of Camptothecin.
The Uses of DBU

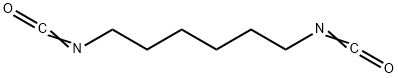
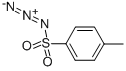
To a suspension of the SM (0.665 g, 1.66 mmol) in THF (10 mL) at RT under Ar was added DPPA (0.400 mL, 1.83 mmol) and DBU (0.297 mL, 1.99 mmol). The resulting mixture was stirred at RT for 18 h. Additional DPPA (0.200 mL, 0.916 mmol) and DBU (0.150 mL, 1.00 mmol) was added and the mixture was stirred at RT for 3 h. The reaction mixture was quenched with sat aq NaHCO3 (30 mL). The aq mixture was extracted EtOAc (2 x 30 mL). The combined organics were dried (Na2SO4) and concentrated to a yellow gum. The material (1.26 g) was purified by Prep LC (2-5% MeOH/DCM) to provide the product as an off-white solid. [370 mg, 52%]
The Uses of DBU
DBU is used in organic synthesis as a catalyst, a complexing ligand, and a non-nucleophilic base. It is used as a protecting agent for the synthesis of cephalosporin and as a catalyst for polyurethane.
What are the applications of Application
1,8-Diazabicyclo[5.4.0]undec-7-ene is an amidine base used for dehydrohalogenation reactions to olefins
General Description
We are committed to bringing you Greener Alternative Products, which adhere to one or more of The 12 Principles of Greener Chemistry. This product has been enhanced for catalytic efficiency. Click here for more information.
Flammability and Explosibility
Non flammable
Synthesis
The synthesis of DBU is as follows:As a base, sodium hydroxide was used at a concentration of 25% by weight and 2.3 times molar equivalent. A stirrer was placed in a 100 mL screw tube, and 11.5 g (50 mmol) of DBU hydrochloride, 8.5 g of toluene and 18.5 g (116 mmol) of 25% sodium hydroxide aqueous solution were charged at room temperature. It was placed on a magnetic stirrer and mixed for 1 hour with stirring. The mixture was separated with a 100 mL separatory funnel to obtain 18.8 g of the upper layer containing DBU and 18.4 g of the lower layer of the aqueous layer. The upper layer portion was analyzed by gas chromatography and contained 31.9% by weight of DBU, and the yield was 6.0 g (39.4 mmol) in a yield of 78.8%.

Purification Methods
Fractionally distil DBU under vacuum. Also purify it by chromatography on Kieselgel and eluting with CHCl3/EtOH/25% aqueous NH3 (15:5:2) and checking by IR and MS. [Oediger et al. Chem Ber 99 2012 1962, Angew Chem, Int Ed Engl 6 76 1967, Guggisberg et al. Helv Chim Acta 61 1057 1978, Beilstein 23/5 V 271.]
Properties of DBU
| Melting point: | -70 °C |
| Boiling point: | 80-83 °C0.6 mm Hg(lit.) |
| Density | 1.019 g/mL at 20 °C(lit.) |
| vapor pressure | 5.3 mm Hg ( 37.7 °C) |
| refractive index | n |
| Flash point: | >230 °F |
| storage temp. | Store below +30°C. |
| solubility | soluble |
| form | Liquid |
| appearance | Colorless to light yellow liquid |
| pka | 13.28±0.20(Predicted) |
| color | Clear colorless to light yellow |
| Odor | Unpleasant |
| PH Range | 12.8 at 10 g/l at 20 °C |
| PH | 12.8 (10g/l, H2O, 20℃) |
| explosive limit | 1.1-6.5%(V) |
| Water Solubility | soluble |
| Sensitive | Air Sensitive |
| BRN | 508906 |
| Stability: | Stable. Incompatible with strong oxidizing agents, acids, acid chlorides, acid anhydrides. |
| CAS DataBase Reference | 6674-22-2(CAS DataBase Reference) |
| NIST Chemistry Reference | 1,8-diazabicyclo [5.4.0]undec-7-ene(6674-22-2) |
| EPA Substance Registry System | Pyrimido[1,2-a]azepine, 2,3,4,6,7,8,9,10-octahydro- (6674-22-2) |
Safety information for DBU
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H290:Corrosive to Metals H301:Acute toxicity,oral H314:Skin corrosion/irritation H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P234:Keep only in original container. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for DBU
| InChIKey | GQHTUMJGOHRCHB-UHFFFAOYSA-N |
DBU manufacturer
JSK Chemicals
Dodhia Group
Anand Agencies
ASM Organics
CLEARSYNTH LABS LTD.
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![1,8-DIAZABICYCLO[5.4.0]UNDEC-7-ENE, COMPOUND WITH P-TOLUENESULFONIC ACID (1:1)](https://img.chemicalbook.in/CAS/GIF/51376-18-2.gif)
![1,8-DIAZABICYCLO[5.4.0]UNDEC-7-ENE, COMPOUND WITH PHENOL (1:1)](https://img.chemicalbook.in/CAS/GIF/57671-19-9.gif)
![1,8-DIAZABICYCLO[5.4.0]UNDEC-7-ENE, COMPOUND WITH 2-ETHYLHEXANOIC ACID (1:1),1,8-Diazabicyclo[5.4.0]undec-7-ene 2-ethylhexanoate](https://img.chemicalbook.in/CAS/GIF/33918-18-2.gif)
![6-(DIBUTYLAMINO)-1,8-DIAZABICYCLO[5.4.0]UNDEC-7-ENE](https://img.chemicalbook.in/CAS/GIF/106847-76-1.gif)
![1,8-DIAZABICYCLO[5.4.0]UNDEC-7-ENE HYDROTRIBROMIDE](https://img.chemicalbook.in/CAS/GIF/138666-59-8.gif)
You may like
-
![1,8-Diazabicyclo[5.4.0]undec-7-ene(DBU) High quality high purity, size packaging, large supply](https://img.chemicalbook.in//ProductImageEN/2022-05/Raw/1e9fe761-f25e-4852-bc53-ff7e3fdd811f.jpg) 1,8-Diazabicyclo[5.4.0]undec-7-ene(DBU) High quality high purity, size packaging, large supplyView Details
1,8-Diazabicyclo[5.4.0]undec-7-ene(DBU) High quality high purity, size packaging, large supplyView Details
6674-22-2 -
![1,8 Diazabicyclo[5.4.0]undec-7-ene [DBU] 6674-22-2 99%](https://img.chemicalbook.in//ProductImageIndia/2024-03/Raw/694fde07-b85c-49bf-ab8f-551942ca22e8.png) 1,8 Diazabicyclo[5.4.0]undec-7-ene [DBU] 6674-22-2 99%View Details
1,8 Diazabicyclo[5.4.0]undec-7-ene [DBU] 6674-22-2 99%View Details
6674-22-2 -
 1,8-Diazabicycloundecene CAS 6674-22-2View Details
1,8-Diazabicycloundecene CAS 6674-22-2View Details
6674-22-2 -
![1,8-Diazabicyclo[5.4.0]-7-undecene CAS 6674-22-2](https://img.chemicalbook.in//Content/image/CP5.jpg) 1,8-Diazabicyclo[5.4.0]-7-undecene CAS 6674-22-2View Details
1,8-Diazabicyclo[5.4.0]-7-undecene CAS 6674-22-2View Details
6674-22-2 -
![1,8-Diazabicyclo[5.4.0]undec-7-ene CAS](https://img.chemicalbook.in//Content/image/CP5.jpg) 1,8-Diazabicyclo[5.4.0]undec-7-ene CASView Details
1,8-Diazabicyclo[5.4.0]undec-7-ene CASView Details -
![1,8-Diazabicyclo[5.4.0]undec-7-ene 99%](https://img.chemicalbook.in//ProductImageIndia/2024-03/Raw/626069ab-d1ef-420f-ae90-dc2dcf35d0bd.png) 1,8-Diazabicyclo[5.4.0]undec-7-ene 99%View Details
1,8-Diazabicyclo[5.4.0]undec-7-ene 99%View Details -
![1,8-Diazabicyclo[5.4.0]undec-7-ene CAS](https://img.chemicalbook.in//Content/image/CP5.jpg) 1,8-Diazabicyclo[5.4.0]undec-7-ene CASView Details
1,8-Diazabicyclo[5.4.0]undec-7-ene CASView Details -
![1,8-Diazabicyclo[5.4.0]undec-7-ene CAS](https://img.chemicalbook.in//Content/image/CP5.jpg) 1,8-Diazabicyclo[5.4.0]undec-7-ene CASView Details
1,8-Diazabicyclo[5.4.0]undec-7-ene CASView Details