4-Methylpyridine
Synonym(s):4-Methylpyridine;4-Picoline
- CAS NO.:108-89-4
- Empirical Formula: C6H7N
- Molecular Weight: 93.13
- MDL number: MFCD00006440
- EINECS: 203-626-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-11-27 15:07:19
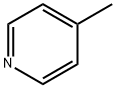
What is 4-Methylpyridine?
Chemical properties
4-Methylpyridine is a Colorless to light yellow liquid with an obnoxious, sweetish odor. soluble in water, ethanol and ether.
Occurrence
4-Methylpyridine is released by energy-related processes. It is present in coal gassification wastewater (Pellizzari et al 1979), the environment of coke ovens (Naizer and Mashek 1974) and in waters from shale oil waste production (Dobson et al 1985; Hawthorne et al 1985; Leenheer et al 1982). It is also present in coal tar (HSDB, 1988), cigarette smoke (Brunneman et al 1978; IARC 1976) and pyroligneous liquids from woods (Yasuhara and Sugiwara 1987). Methods for the biological treatment of wastewaters containing 4-methylpyridine have been developed (Roubickova 1986) and its movement through (Leenheer and Stuber 1981) and degradation (Sims and Somners 1985) in soils examined.
The Uses of 4-Methylpyridine
4-Methylpyridine is used to manufacture isonicotinic acid and derivatives, in waterproofing agents for fabric, and as a solvent for resins, pharmaceuticals, dyestuffs, rubber accelerators, and pesticides. It is also used as a catalyst and curing agent.
The Uses of 4-Methylpyridine
4-Methylpyridine was used in the preparation of a 1,2-dihydropyridide derivative.
What are the applications of Application
4-Picoline is a neutral N-donor ligand
Definition
ChEBI: 4-methylpyridine is a methylpyridine in which the methyl substituent is at position 4.
Production Methods
Currently, 4-methylpyridine is produced by vapor-phase condensation of acetaldehyde and ammonia (3:1) with subsequent isolation of 4-methylpyridine from the reaction mixture. Reactants are exposed to dehydration-dehydrogenation catalysis such as lead oxide, copper oxide on alumina, thorium oxide, zinc oxide or cadmium oxide on silica-alumina, or cadmium fluoride on silica-magnesia at 400-500°C. This results in a 60% yield of 4-methylpyridine which is isolated by fractional distillation (USEPA 1982). Another production method involves the isolation from by-products of coking operations. The crude pyridine extracts come from noncondensable and condensable coke-oven gasses that have been dehydrated and separated by fractional distillation, but only 45% of 4-methylpyridine is obtained (USEPA 1982). 4-Methylpyridine also can be isolated from a dry distillation of bones or coal (Hawley 1977).
Synthesis Reference(s)
Chemical and Pharmaceutical Bulletin, 6, p. 467, 1958 DOI: 10.1248/cpb.6.467
General Description
4-methyl pyridine is a colorless moderately volatile liquid. (NTP, 1992)
Air & Water Reactions
Highly flammable.
Reactivity Profile
4-Methylpyridine neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen may be generated in combination with strong reducing agents, such as hydrides.
Health Hazard
Data indicates that 4-methylpyridine is moderately toxic when administered orally and very toxic when given dermally and intraperitoneally (Smith 1982). Symptoms include occasional diarrhea, weight loss, anemia, and occular and facial paralysis.
Flammability and Explosibility
Flammable
Industrial uses
4-Methylpyridine is used as a water-proofing agent for fabrics; as solvents for resins; in the synthesis of pharmaceuticals, dyestuffs, rubber accelerators, pesticides and laboratory reagent; as a catalyst; and as a curing agent (Hawley 1977; Windholz et al 1983). It is used for the synthesis of pharmaceuticals, especially isoniazid (USEPA 1982) and also for the production of 4-vinylpyridine to improve dyeability (USEPA 1982).
Safety Profile
Poison by ingestion and intraperitoneal routes. Moderately toxic by sktn contact. Mildly toxic by inhalation. A severe skin and eye irritant. Flammable liquid when exposed to heat, flames, oxidizers. To fight fire, use alcohol foam. When heated to decomposition it emits toxic fumes of NOx.
Potential Exposure
(o-isomer); Suspected reprotoxic hazard, Primary irritant (w/o allergic reaction), (m-isomer): Possible risk of forming tumors, Primary irritant (w/o allergic reaction). Picolines are used as intermediates in pharmaceutical manufacture, pesticide manufacture; and in the manufacture of dyes and rubber chemicals. It is also used as a solvent.
Shipping
UN2313 Picolines, Hazard Class: 3; Labels: 3-Flammable liquid.
Purification Methods
It can be purified as for 2-methylpyridine. Biddescombe and Handley's method (above) for 3-methylpyridine is also applicable. Lidstone [J Chem Soc 242 1940] purified it via the oxalate (m 137-138o) by heating 100mL of 4-methylpyridine to 80o and adding slowly110g of anhydrous oxalic acid, followed by 150mL of boiling EtOH. After cooling and filtering, the precipitate is washed with a little EtOH, then recrystallised from EtOH, dissolved in the minimum quantity of water and distilled with excess 50% KOH. The distillate is dried with solid KOH and again distilled. Hydrocarbons can be removed from 4-methylpyridine by converting the latter to its hydrochloride, crystallising from EtOH/diethyl ether, regenerating the free base by adding alkali and distilling. As a final purification step, 4-methylpyridine can be fractionally crystallised by partial freezing to effect a separation from 3-methylpyridine. Contamination with 2,6-lutidine is detected by its strong absorption at 270nm. The hydrochloride has m 161o, and the picrate has m 167o(from Me2CO, EtOH or H2O). [Beilstein 20 III/IV 2732, 20/5 V 543.]
Incompatibilities
Vapors may form explosive mixture with air. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Attacks copper and its alloys.
Properties of 4-Methylpyridine
| Melting point: | 2.4 °C (lit.) |
| Boiling point: | 145 °C (lit.) |
| Density | 0.957 g/mL at 25 °C (lit.) |
| vapor density | 3.2 (vs air) |
| vapor pressure | 4 mm Hg ( 20 °C) |
| refractive index | n |
| Flash point: | 134 °F |
| storage temp. | Store below +30°C. |
| solubility | alcohol: soluble(lit.) |
| form | Liquid |
| pka | 6.02(at 20℃) |
| color | Clear light yellow |
| Odor | Obnoxious, Sweetish |
| explosive limit | 1.3-8.7%(V) |
| Water Solubility | soluble |
| Sensitive | Air Sensitive & Hygroscopic |
| Merck | 14,7402 |
| BRN | 104586 |
| Dielectric constant | 12.279999999999999 |
| CAS DataBase Reference | 108-89-4(CAS DataBase Reference) |
| NIST Chemistry Reference | Pyridine, 4-methyl-(108-89-4) |
| EPA Substance Registry System | 4-Methylpyridine (108-89-4) |
Safety information for 4-Methylpyridine
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H226:Flammable liquids H311:Acute toxicity,dermal H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 4-Methylpyridine
| InChIKey | FKNQCJSGGFJEIZ-UHFFFAOYSA-N |
4-Methylpyridine manufacturer
JSK Chemicals
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 4-Picoline 98%View Details
4-Picoline 98%View Details -
 108-89-4 99%View Details
108-89-4 99%View Details
108-89-4 -
 4-Methylpyridine CAS 108-89-4View Details
4-Methylpyridine CAS 108-89-4View Details
108-89-4 -
 4-Methyl pyridine CAS 108-89-4View Details
4-Methyl pyridine CAS 108-89-4View Details
108-89-4 -
 4-Picoline CAS 108-89-4View Details
4-Picoline CAS 108-89-4View Details
108-89-4 -
 4-METHYL PYRIDINE For Synthesis CAS 108-89-4View Details
4-METHYL PYRIDINE For Synthesis CAS 108-89-4View Details
108-89-4 -
 4-Methylpyridine CAS 108-89-4View Details
4-Methylpyridine CAS 108-89-4View Details
108-89-4 -
 Gamma Picoline (cas no 108-89-4), 190kgView Details
Gamma Picoline (cas no 108-89-4), 190kgView Details
108-89-4
