Cobalt chloride hexahydrate
Synonym(s):Cobalt dichloride;Cobalt(II) chloride hexahydrate;Cobaltous chloride hexahydrate
- CAS NO.:7791-13-1
- Empirical Formula: Cl2CoH2O
- Molecular Weight: 147.85
- MDL number: MFCD00149652
- EINECS: 616-574-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-02-25 20:54:18
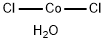
What is Cobalt chloride hexahydrate?
Chemical properties
Cobalt(II) chloride hexahydrate takes the form of purple crystals. Upon heating, it loses its water of crystallisation and decomposes into blue crystals of anhydrous cobalt(II) chloride. Soluble in water, soluble in ethanol, acetone and ether.
Cobalt chloride hexahydrate is utilized in commercial applications such as electroplating, catalyst preparation, painting on glass and porcelain, and in the manufacture of vitamin B12.
The Uses of Cobalt chloride hexahydrate
Cobalt(II) chloride hexahydrate (CoCl2·6H2O) has been found to be an efficient catalyst for the one-pot synthesis of biscoumarin derivatives through a combination of aromatic aldehydes and 4-hydroxycoumarin in aqueous media at 70°C. Cobalt chloride hexahydrate can be used as an inducer of HIF-1 production used to study apoptotic effects in HepG2 cells. It is also used as APHA color standard.
What are the applications of Application
Cobalt(II) chloride hexahydrate is an inducer of HIF-1 production used to study apoptotic effects in HepG2 cells
Definition
ChEBI: Cobalt chloride hexahydrate is a hydrate of cobalt chloride containing cobalt (in +2 oxidation state), chloride and water moieties in the ratio 1:2:6. It has a role as an allergen. It contains a cobalt dichloride.
What are the applications of Application
Cobalt chloride hexahydrate can be used as Invisible ink; humidity and water indicator; in hygrometers; tempereture indicator in grinding; in electroplating; for painting on glass and porcelain; preparation of catalysts; fertilizer and feed additive; foam stabilizer in beer; as absorbent for military poison gas and ammonia; in manufacture of vitamin B12.
General Description
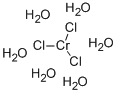
Cobalt(II) chloride hexahydrate has a cobalt ion surrounded by a tetragonal arrangement of chloride ligands. Four water molecules occupy a square plane about cobalt while chloride ions occupy axial positions. Its crystals belong to the monoclinic lattice system.
Purification Methods
A saturated aqueous solution at room temperature is fractionally crystallised by standing overnight. The first half of the material that crystallises in this way is used in the next crystallisation. The process is repeated several times, water being removed in a dry-box using air filtered through glass wool and dried over CaCl2 [Hutchinson J Am Chem Soc 76 1022 1954]. It has also been crystallised from dilute aqueous HCl. The hexahydrate m 86o forms pink to red deliquescent crystals. It loses 4H2O on heating at 52-56o and forms the violet dihydrate which loses a further H2O at 100o to form the violet monohydrate which loses the last H2O at 120-140o to give the pale blue anhydrous deliquescent salt m 735o and b 1049o. A pink solution of CoCl2 in H2O becomes blue on heating to 50o or adding conc HCl which may precipitate the mono or dihydrate. The solid dihydrate gives a blue-purple solution with EtOH. Note: CoCl2 in H2O is a “sympathetic ink”, i.e. writing using an aqueous solution is almost invisible on paper, but becomes blue on warming the paper. On cooling or standing, the writing becomes invisible again. The anhydrous salt is soluble in H2O, EtOH, Et2O, Me2CO and pyridine. [Glemser in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol II p 1515 1965.]
Structure and conformation
The hexahydrate COCl2.6H2O forms monoclinic crystals in which each cobalt atom is surrounded by four water molecules at the corners of a distorted square (Co-H20 = .12) and by two chlorine atoms (Co-Cl = 2.43) to form a distorted octahedron.
The other two water molecules are not bonded directly to cobalt. In the monoclinic dihydrate CoCl2.2H2O, the octahedral symmetry around the cobalt atom is maintained by the sharing of chlorine atoms between two cobalt atoms. In the flattened octahedral structuress there are two Co-Cl distances of 2-450, two of 2.478 and two Co-H2O distances of 2.040.
Properties of Cobalt chloride hexahydrate
| Melting point: | 86 °C |
| Boiling point: | 1049 °C |
| Density | 3.35 |
| vapor pressure | 40 mm Hg ( 0 °C) |
| storage temp. | Store at +5°C to +30°C. |
| solubility | 191g/l |
| form | Solid |
| color | Red-purple |
| Specific Gravity | 1.924 |
| PH | 4.9 (50g/l, H2O, 25℃) |
| Odor | at 100.00?%. odorless |
| Water Solubility | Soluble in water, ether, acetone and alcohol. |
| Sensitive | Hygroscopic |
| Merck | 14,2437 |
| Exposure limits | ACGIH: TWA 0.02 mg/m3 |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 7791-13-1(CAS DataBase Reference) |
| EPA Substance Registry System | Cobalt chloride (CoCl2), hexahydrate (7791-13-1) |
Safety information for Cobalt chloride hexahydrate
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H317:Sensitisation, Skin H334:Sensitisation, respiratory H341:Germ cell mutagenicity H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Cobalt chloride hexahydrate
Cobalt chloride hexahydrate manufacturer
JSK Chemicals
ARRAKIS INDUSTRIES LLP
ASM Organics
Related products of tetrahydrofuran
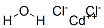

You may like
-
 Cobaltous chloride hexahydrate 98%View Details
Cobaltous chloride hexahydrate 98%View Details -
 Cobalt(II) chloride hexahydrate CAS 7791-13-1View Details
Cobalt(II) chloride hexahydrate CAS 7791-13-1View Details
7791-13-1 -
 Cobalt(II) chloride hexahydrate, For analysis ACS CAS 7791-13-1View Details
Cobalt(II) chloride hexahydrate, For analysis ACS CAS 7791-13-1View Details
7791-13-1 -
 Cobalt(II) chloride hexahydrate, For analysis ACS CAS 7791-13-1View Details
Cobalt(II) chloride hexahydrate, For analysis ACS CAS 7791-13-1View Details
7791-13-1 -
 Cobalt(II) chloride hexahydrate CAS 7791-13-1View Details
Cobalt(II) chloride hexahydrate CAS 7791-13-1View Details
7791-13-1 -
 Cobalt(II) chloride hexahydrate CAS 7791-13-1View Details
Cobalt(II) chloride hexahydrate CAS 7791-13-1View Details
7791-13-1 -
 Cobalt (II) Chloride Hexahydrate extrapure AR CAS 7791-13-1View Details
Cobalt (II) Chloride Hexahydrate extrapure AR CAS 7791-13-1View Details
7791-13-1 -
 Cobalt (II) Chloride Hexahydrate pure CAS 7791-13-1View Details
Cobalt (II) Chloride Hexahydrate pure CAS 7791-13-1View Details
7791-13-1