BERYLLIUM SULFATE TETRAHYDRATE
- CAS NO.:7787-56-6
- Empirical Formula: BeH8O8S
- Molecular Weight: 177.14
- MDL number: MFCD00149156
- EINECS: 629-461-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-07-04 15:24:28
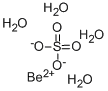
What is BERYLLIUM SULFATE TETRAHYDRATE?
Chemical properties
colourless crystals
Physical properties
Beryllium sulfate is a colorless crystalline solid with a tetragonal crystal system.The tetrahydrate salt loses water of crystallization on heating to about 110°C to form a dihydrate. Upon heating to 400°C, it then forms the anhydrate. Further heating to 550°C causes decomposition of the anhydrate to BeO and SO2 and O2. Beryllium sulfate is soluble in water, tetrahydrate being more soluble in water (30.5 g/100 ml at 30°C) than the anhydrous salt; both are insoluble in alcohol.
The Uses of BERYLLIUM SULFATE TETRAHYDRATE
In Industries it is used for beryllium salt making, ceramics and chemical reagents.
Definition
ChEBI: A hydrate of beryllium sulfate containing beryllium (in +2 oxidation state), sulfate and water moieties in the ratio 1:1:4.
Preparation
Beryllium sulfate may be prepared by
treating an aqueous solution of any beryllium salt
with sulfuric acid, followed by evaporation of the solution
and crystallization. The hydrated product may be
converted to anhydrous salt by heating at 400°C. The
anhydrate may also be prepared by reacting the oxide
with SO2 gas at elevated temperature. Its CAS number
is 13510-49-1. If the solution method is used, a tetrahydrate
forms, (BeOH)2SO4·4H2O whose molecular
weight is 177.1462 g/mol with a CAS number of 7787-56-6.
No major commercial application of berylliumsulfate
is known and it has not been offered for sale.
General Description
White crystalline solid. Sinks in water and dissolves. Odorless. Highly toxic by inhalation and ingestion.
Air & Water Reactions
Water soluble.
Reactivity Profile
Salts, basic, such as BERYLLIUM SULFATE TETRAHYDRATE are generally soluble in water. The resulting solutions contain moderate concentrations of hydroxide ions and have pH's greater than 7.0. They react as bases to neutralize acids. These neutralizations generate heat, but less or far less than is generated by neutralization of the bases in reactivity group 10 (Bases) and the neutralization of amines. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible. Incompatible with acids.
Fire Hazard
Literature sources indicate that BERYLLIUM SULFATE TETRAHYDRATE is probably nonflammable.
Safety Profile
Confirmed carcinogen withexperimental carcinogenic data by inhalation. Deadlypoison by subcutaneous and intravenous routes. Humanmutation data reported. When heated todecomposition it emits very toxic fumes of BeO and SOx.
Purification Methods
It crystallises from weak aqueous H2SO4 and is dried in air.
Properties of BERYLLIUM SULFATE TETRAHYDRATE
| Melting point: | 270℃ |
| Density | 1.713 g/mL at 25 °C(lit.) |
| solubility | insoluble in ethanol |
| form | Lumps |
| color | Colorless, tetragonal crystals |
| Specific Gravity | 1.713 |
| Water Solubility | Freely soluble in water. Insoluble in alcohol |
| Merck | 14,1175 |
| Stability: | Stable. |
| CAS DataBase Reference | 7787-56-6(CAS DataBase Reference) |
| EPA Substance Registry System | Beryllium monosulfate tetrahydrate (7787-56-6) |
Safety information for BERYLLIUM SULFATE TETRAHYDRATE
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H315:Skin corrosion/irritation H317:Sensitisation, Skin H319:Serious eye damage/eye irritation H330:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H372:Specific target organ toxicity, repeated exposure H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for BERYLLIUM SULFATE TETRAHYDRATE
| InChIKey | DIMYTQPLZWDZFE-UHFFFAOYSA-L |
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
You may like
-
 BERYLLIUM SULPHATE TETRAHYDRATE 99%View Details
BERYLLIUM SULPHATE TETRAHYDRATE 99%View Details
7787-56-6 -
 Beryllium sulphate CAS 7787-56-6View Details
Beryllium sulphate CAS 7787-56-6View Details
7787-56-6 -
 Beryllium sulphate CAS 7787-56-6View Details
Beryllium sulphate CAS 7787-56-6View Details
7787-56-6 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
