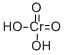
Potassium dichromate
Synonym(s):Potassium bichromate;Potassium bichromate, Potassium pyrochromate;Potassium dichromate
- CAS NO.:7778-50-9
- Empirical Formula: Cr2K2O7
- Molecular Weight: 294.1846
- MDL number: MFCD00010944
- EINECS: 231-906-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
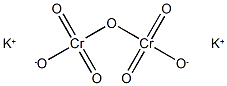
What is Potassium dichromate?
Chemical properties
Also known as potassium bichromate and red potassium chromate, K2Cr2O7 is poisonous,yellowish-red crystals with a metallic taste that is soluble in water,insoluble in alcohol,that melt at 396℃;and decompose at 500℃. Used as an oxidizing agent and analytical reagent,and in explosives, matches, and electroplating.
Chemical properties
Potassium chromate(VI) is a yellow crystalline solid.
The Uses of Potassium dichromate
Potassium bichromate is also known as potassium dichromate, this bright orange crystal was made by the acidification of potassium chromate. It is toxic and an oxidant and is soluble in water but not in alcohol. In 1839 Mungo Ponton sensitized paper with a solution of potassium bichromate to print-out images in the sun. The most important use of potassium bichromate was used to make colloids sensitive to light for a variety of processes such as the photoglyphic engraving process, the carbon process, the gum bichromate process, and in the preparation of the gelatin relief used to make lead molds for the Woodburytype.
The Uses of Potassium dichromate
In tanning leather, dyeing, painting, decorating porcelain, printing, photolithography, pigment-prints, staining wood, pyrotechnics, safety matches; for bleaching palm oil, wax, and sponges; waterproofing fabrics; as oxidizer in the manufacture of organic chemicals; in electric batteries; as depolarizer for dry cells. As corrosion inhibitor in preference to sodium dichromate where lower soly is advantageous. Pharmaceutic aid (oxidizing agent).
The Uses of Potassium dichromate
Used (1) in matches, (2) in leather tanning and in the textile industry, (3) as a source of chromate, (4) in pyrotechnics, (5) in colored glass, (6) as an important laboratory reagent, (7) in blueprint developing, and (8) in wood preservation formulations.
What are the applications of Application
Potassium dichromate is A strong oxidizing agent used to determine ethanol concentrations
Definition
ChEBI: A potassium salt that is the dipotassium salt of dichromic acid.
General Description
Orange red crystals. Denser than water and soluble in water. No distinctive odor. May severely irritate the eyes and respiratory tract. Avoid contact with organic materials. Noncombustible. Used in pyrotehnic displays with tungsten and iron.
Air & Water Reactions
Soluble in water.
Reactivity Profile
Potassium or sodium dichromate reacts explosively with hydrazine [Mellor 11:234. 1946-47]. A drop of anhydrous hydroxylamine on powdered potassium dichromate produces a violent explosion [Mellor 8:293. 1946-47].
Hazard
Toxic by ingestion and inhalation. Dan- gerous fire risk in contact with organic materials. Strong oxidizing agent.
Health Hazard
Highly corrosive to skin and mucous membranes. If ingested, causes violent gastroenteritis, peripheral vascular collapse, vertigo, muscle cramps, coma, and (later) toxic nephritis with glycosuria. Allergic reactions may also occur.
Fire Hazard
Behavior in Fire: May decompose, generating oxygen. Supports the combustion of other materials.
Industrial uses
This material, K2Cr2O7, decomposes at 500°C.Bright yellowish-red crystals are soluble andpoisonous. Sometimes potassium chromate,K2Cr2O4, and the dichromate are utilized inceramics as coloring agents.
Potassium dichromate is used in glass foraventurine effects. It is said that 20 or 21 partsto 100 parts sand will give a chrome aventurine.This glass is characterized by glittering metallicscales of chromium oxide. Potassiumdichromate is also used in glass to give a greencolor. However, it has been shown that it may cause considerable trouble by formation ofblack, chrome corundum crystals in the glass.Air-floated chromite is suggested to avoid thisproblem.
Potassium dichromate is used in glazes toproduce chrome-tin pinks, low-fire reds, greens,and purplish-red colors.
Safety Profile
Human poison by ingestion. An experimental poison by ingestion, intraperitoneal, intravenous, and subcutaneous routes. Human mutation data reported. An experimental teratogen. Other experimental reproductive effects. Flammable by chemical reaction. A powerful oxidzer. Explosive reaction with hydrazine. Reacts violently or ignites with H2SO4+ acetone, hydroxylamine, ethylene glycol (above 100℃). Forms pyrotechnic mixtures with boron + dion, iron (igmtes at 1090℃), tungsten (igmtes at 1700℃). Reacts with sulfuric acid to form the strong oxidant chromic acid. Used in photomechanical processing, chrome pigment production, and wool preservation methods. When heated to decomposition it emits toxic fumes of K2O. See also CHROMIUM COMPOUNDS.
Potential Exposure
Potassium chromate is used in printing: photomechanical processing; chrome-pigment production; and wool preservative methods; to make dyes, pigments, inks and enamels; as an oxidizing agent; analytical reagent; in electroplating; explosives.
Shipping
UN1479 Oxidizing solid, n.o.s., Hazard Class: 5.1; Labels: 5.1-Oxidizer, Technical Name Required. UN3288 Toxic solids, inorganic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required
Purification Methods
Crystallise it from water (g/mL) between 100o and 0o and dry it under vacuum at 156o. (Possible CARCINOGEN.)
Incompatibilities
A powerful oxidizer. Violent reactions with combustibles, organics, powdered metals; or easily oxidizable substances. Contact with hydroxylamine, hydrazine causes explosion.
Properties of Potassium dichromate
| Melting point: | 398 °C (lit.) |
| Boiling point: | 82 °C |
| Density | 7.14 g/mL at 25 °C(lit.) |
| Flash point: | 50 °F |
| storage temp. | Store at RT. |
| solubility | H2O: 0.1 M at 20 °C, clear, orange |
| form | powder |
| color | Orange-red |
| Specific Gravity | 2.676 |
| PH | 3.5-5.0 (25℃, 0.1M in H2O) |
| Water Solubility | 125 g/L (20 ºC) |
| Merck | 14,7627 |
| Exposure limits | ACGIH: TWA 0.0002 mg/m3; STEL 0.0005 mg/m3 (Skin) OSHA: Ceiling 0.1 mg/m3 NIOSH: IDLH 15 mg/m3; TWA 0.0002 mg/m3 |
| CAS DataBase Reference | 7778-50-9(CAS DataBase Reference) |
| EPA Substance Registry System | Potassium dichromate (7778-50-9) |
Safety information for Potassium dichromate
| Signal word | Danger |
| Pictogram(s) |
 Flame Over Circle Oxidizers GHS03  Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H272:Oxidising liquids;Oxidising solids H301:Acute toxicity,oral H312:Acute toxicity,dermal H314:Skin corrosion/irritation H317:Sensitisation, Skin H330:Acute toxicity,inhalation H334:Sensitisation, respiratory H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H340:Germ cell mutagenicity H350:Carcinogenicity H372:Specific target organ toxicity, repeated exposure H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Potassium dichromate
| InChIKey | KMUONIBRACKNSN-UHFFFAOYSA-N |
Potassium dichromate manufacturer
C.H. Chemicals
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 Potassium dichromate, For ACS analysis CAS 7778-50-9View Details
Potassium dichromate, For ACS analysis CAS 7778-50-9View Details
7778-50-9 -
 Potassium dichromate, For ACS analysis CAS 7778-50-9View Details
Potassium dichromate, For ACS analysis CAS 7778-50-9View Details
7778-50-9 -
 Potassium dichromate, For ACS analysis CAS 7778-50-9View Details
Potassium dichromate, For ACS analysis CAS 7778-50-9View Details
7778-50-9 -
 Potassium dichromate, Standardized Solution CAS 7778-50-9View Details
Potassium dichromate, Standardized Solution CAS 7778-50-9View Details
7778-50-9 -
 Potassium dichromate, Standardized Solution CAS 7778-50-9View Details
Potassium dichromate, Standardized Solution CAS 7778-50-9View Details
7778-50-9 -
 Potassium dichromate solution CASView Details
Potassium dichromate solution CASView Details -
 Potassium dichromate solution CASView Details
Potassium dichromate solution CASView Details -
 Potassium dichromate solution CASView Details
Potassium dichromate solution CASView Details
