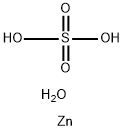
Copper(II) sulfate pentahydrate
Synonym(s):Copper(II) sulfate pentahydrate;Cupric sulfate pentahydrate;Cupric sulfate standard;Sulfuric acid, copper(II) salt (1:1) pentahydrate;Blue Vitriol
- CAS NO.:7758-99-8
- Empirical Formula: CuH10O9S
- Molecular Weight: 249.68
- MDL number: MFCD00149681
- EINECS: 616-477-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-11-20 17:40:16
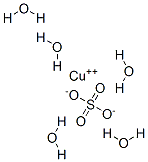
What is Copper(II) sulfate pentahydrate?
Description
Copper(II) sulfate pentahydrate is known as blue vitriol. It is an odorless blue crystal that readily dissolves in water. It is also soluble in methanol, glycerol and slightly soluble in ethanol. The highly toxic, non-combustible has a nauseating metallic taste and turns white when dehydrated. Structurally, in the pentahydrate molecule, each copper(II) ions is surrounded by four water molecules in the corners and the fifth water molecule is attached by hydrogen bonding. Copper (II) sulfate has many applications including preparation of Bordeaux mixture, a fungicide preparation. Electroplating, timber preservation and textile industry use copper (II) sulfate.
Chemical properties
Copper sulfate is a greenish-white crystalline solid; the pentahydrate is Blue powder or granules, or ultramarine crystalline solid.
The Uses of Copper(II) sulfate pentahydrate
Copper (II) sulfate pentahydrate salt may be used for the fabrication of copper nanoparticles by chemical reduction. The pentahydrate salt of copper may be used as a catalyst for the conversion of aromatic aldehydes to primary amides via aldoximes. Reduced graphene oxide-supported copper nanoparticles (rGO/Cu NPs) may be prepared by copper (II) sulfate pentahydrate and graphitic precursors. An aqueous electrolytic bath containing CuSO4.5H2O as one of the constituents was used for the preparation of Cu2ZnSnS4 (CZTS) thin film solar cell. Ferric chloride hexahydrate (FeCl3.6H2O) and copper(II) sulfate pentahydrate (CuSO4.5H2O) may be used to fabricate Fe-Cu binary oxide sorbents for arsenic removal applications.
The Uses of Copper(II) sulfate pentahydrate
Used as a source of Cu2+ ions
Definition
ChEBI: Copper(II) sulfate pentahydrate is the pentahydrate of copper(2+) sulfate. A bright blue crystalline solid. It is a hydrate and a metal sulfate. It contains a copper(II) sulfate.
Definition
A green mineral consisting of copper(II) carbonate and hydroxide (CuCO3.Cu(OH)2). It is used as an ore and a pigment.
Definition
malachite: Apresumptive test for blood. Thereagent is the dye leucomalachitegreen dissolved in water along withsodium perborate (NaBO3). A bluegreencolour indicates a positive result.
General Description
Blue crystalline granules or powder. Melting point 110°C (with decomposition). Non-combustible. Nauseating metallic taste. Odorless. White when dehydrated.
Air & Water Reactions
Slowly effloresces in air. Water soluble.
Reactivity Profile
Copper sulfate pentahydrate can be dehydrated by heating. Serves as a weak oxidizing agent. Causes hydroxylamine to ignite. Gains water readily. The hydrated salt is vigorously reduced by hydroxylamine [Mellor 8:292(1946-1947)]. Both forms are incompatible with finely powdered metals. Both are incompatible with magnesium, corrode steel and iron, may react with alkalis, phosphates, acetylene gas, hydrazine, or nitromethane, and may react with beta-naphthol, propylene glycol, sulphathiazole and triethanolamine if the pH exceeds 7 . Both act as acidic salts, corrode metals and irritate tissues.
Fire Hazard
Literature sources indicate that Copper sulfate pentahydrate is nonflammable.
Potential Exposure
Copper sulfate is used as intermediate and wood preservative; also used in production of copper compounds; to detect and to remove trace amounts of water from alcohols and organic compounds; as a fungicide and algicide; in veterinary medicine and others.
Shipping
UN3288 Toxic solids, inorganic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required. UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.
Incompatibilities
Aqueous solution is an acid. May form explosive materials on contact with acetylene and nitromethane. Incompatible with strong bases; hydroxylamine, magnesium; zirconium, sodium hypobromite, hydrazine.
Waste Disposal
Copper-containing soluble wastes can be concentrated through the use of ion exchange, reverse osmosis, or evaporators to the point where copper can be electrolytically removed and sent to a reclaiming firm. If recovery is not feasible, the copper can be precipitated through the use of caustics and the sludge deposited in a chemical waste landfill Add soda ash to waste CuSO4 solution; let stand 24 hours. Decant and neutralize solution before flushing to sewer. Landfill sludge.
Properties of Copper(II) sulfate pentahydrate
| Melting point: | 110 °C (dec.)(lit.) |
| Density | 2.284 |
| vapor pressure | 7.3 mm Hg ( 25 °C) |
| storage temp. | Store at +5°C to +30°C. |
| solubility | 320 g/L (20°C) |
| form | Solid |
| color | fine blue crystals |
| Specific Gravity | 2.284 |
| PH | 3.5-4.5 (25℃, 50mg/mL in H2O) |
| Odor | blue cryst. or cryst. gran. or powd., odorless |
| Water Solubility | 320 g/L (20 ºC) |
| Merck | 14,2653 |
| Exposure limits | ACGIH: TWA 1 mg/m3 NIOSH: IDLH 100 mg/m3; TWA 1 mg/m3 |
| CAS DataBase Reference | 7758-99-8(CAS DataBase Reference) |
| EPA Substance Registry System | Copper sulfate pentahydrate (7758-99-8) |
Safety information for Copper(II) sulfate pentahydrate
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H318:Serious eye damage/eye irritation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Copper(II) sulfate pentahydrate
Copper(II) sulfate pentahydrate manufacturer
Techno Pharmchem
Jaiswal S Cyber Shop
PHD Chemicals
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 Copper sulphate pentahydrate 99%View Details
Copper sulphate pentahydrate 99%View Details -
 Cupric sulfate pentahydrate 98%View Details
Cupric sulfate pentahydrate 98%View Details -
 Cupric sulfate pentahydrate 99%View Details
Cupric sulfate pentahydrate 99%View Details -
 Copper(II) sulfate, pentahydrate CAS 7758-99-8View Details
Copper(II) sulfate, pentahydrate CAS 7758-99-8View Details
7758-99-8 -
 Copper(II) sulfate, pentahydrate CAS 7758-99-8View Details
Copper(II) sulfate, pentahydrate CAS 7758-99-8View Details
7758-99-8 -
 Copper(II) sulfate pentahydrate, 98% 99%View Details
Copper(II) sulfate pentahydrate, 98% 99%View Details -
 99% Copper Sulphate Pentahydrate, Packaging Type: Bag, Packaging Size: 50 kgView Details
99% Copper Sulphate Pentahydrate, Packaging Type: Bag, Packaging Size: 50 kgView Details
7758-98-7 -
 Copper Sulphate PentahydrateView Details
Copper Sulphate PentahydrateView Details
7758-98-7
