BAY 11-7082
Synonym(s):(E)-3-(4-Methylphenylsulfonyl)-2-propenenitrile;(E)3-[(4-Methylphenyl)sulfonyl]-2-propenenitrile;BAY 11-7082 - CAS 19542-67-7 - Calbiochem
- CAS NO.:19542-67-7
- Empirical Formula: C10H9NO2S
- Molecular Weight: 207.25
- MDL number: MFCD00712162
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
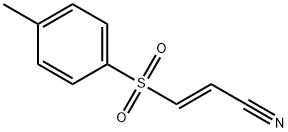
What is BAY 11-7082?
Description
BAY 11-7082 (19542-67-7)?inhibits cytokine-induced IκB-α phosphorylation via inhibition of IκB Kinase which results in inhibition of NFκB .1 Inhibits anchorage-independent growth of mammary epithelial cells induced with 4-hydroxyestradiol via inhibition of NFκB activation.2 Inhibits IFNα production and blocks nuclear translocation of IRF7 in plasmacytoid dendritic cells.3? Facilitates wound healing by inhibiting TNFα-induced MMP expression.4 A useful tool for probing the involvement of NFκB in physiological and pathophysiological processes.5? Cell permeable.
The Uses of BAY 11-7082
Bay 11-7082 has been used as:
- a nuclear factor-kappa B (NF-kB) inhibitor to verify the action of the NF-kB signaling pathway in the production of interleukin (IL)-8
- a nuclear factor-kappa B (NF-kB) inhibitor to study the role of NF-kB activation in Mycoplasma hyorhinis -induced epithelial-mesenchymal transition (EMT) and cell migration
- a nod-like receptor family pyrin domain containing 3 (NLRP3) selective inhibitor to examine its effects on liver inflammation in mice after hematopoietic stem cell transplantation
What are the applications of Application
BAY 11-7082 is an anti-inflammatory agent and NFκB inhibitor
Definition
ChEBI: A nitrile that is acrylonitrile in which the hydrogen located beta,trans to the cyano group is replaced by a tosyl group. It is an inhibitor of cytokine-induced IkappaB-alpha phosphorylation i cells.
General Description
Potential anti-inflammatory agent that selectively and irreversibly inhibits the TNF-α-inducible phosphorylation of IκBα (IC50 = 10 μM), resulting in a decreased expression of NF-κB and of adhesion molecules. Does not affect constitutive IκBα autophosphorylation. Inhibits TNF-α-induced surface expression of the endothelial-leukocyte cell adhesion molecules E-selectin, VCAM-1, and ICAM-1. A 100 mM (10 mg/483 μl) solution of BAY 11-7082 (Cat. No. 196871) in DMSO is also available is also available.
Biological Activity
Irreversible inhibitor of TNF- α -stimulated I κ B α phosphorylation (IC 50 ~ 10 μ M); leads to decreased NF- κ B and subsequent decreased expression of adhesion molecules. Also reversibly activates MAP kinases and stimulates apoptosis.
Biochem/physiol Actions
Bay 11-7082 acts as a selective inhibitor for nod-like receptor family pyrin domain containing 3 (NLRP3) inflammasome pathway. In addition, to the inhibition of nuclear factor-kappa B (NF-kB), Bay 11-7082 also triggers apoptosis in anucleated erythrocytes, human T-cell leukemia virus type I (HTLV-I)-infected T-cell lines and primary adult T-cell leukemia cells.
Enzyme inhibitor
This protein kinase inhibitor (FW = 207.31 g/mol; CAS 19542-67-7; lmax = 251 nm), also named 3-[(4-methylphenyl)sulfonyl]-(2E)-propenenitrile, targets NF-κB activation, selectively and irreversibly blocking TNF-α- induced phosphorylation of IκB-α without affecting phosphorylation of constitutive IκB-α. Mechanism of Inhibitory Action: NF-κB transcription factor regulates expression of inflammatory cytokines, various chemokines and immunoreceptors, as well as cell adhesion molecules. When stationed within the cytoplasm, NF-κB is kept inactive through its binding to the inhibitory factor IκB; however, certain stimuli result in IκB phosphorylation and ubiquitin-mediated degradation, freeing NF-κB for translocation to the nucleus. In endothelial cells, IκB-α phosphorylation and degradation occur within 15 min of TNFα treatment, allowing NF-κB to translocate to the nucleus to activate gene expression. Treatment of humnan vascular endothelial cells (HUVEC) with TNFα results in rapid loss of IκB-α from the cytoplasm. BAY11-7082 stabilizes IκB-α in a dose-dependent manner (IC50 ≈ 10 μM). There is a clear correlation between the concentration of drug that stabilizes IκB-α, the concentration that inhibits nuclear levels of NF-κB, and the concentration that inhibits adhesion molecule expression. More recent studies demonstrate that BAY 11- 7082 prevents ubiquitin conjugation to Ubc13 and UbcH7 by forming a covalent adduct with their cysteine residues via Michael addition at the C-3 atom of BAY 11-7082, followed by the release of 4-methylbenzenesulfinate. BAY 11-7082 stimulated Lys48-linked polyubiquitin chain formation in cells and protected Hypoxia-Inducible Factor-1α (HIF1α) from proteasomal degradation, suggesting it inhibits the proteasome. These results indicate that the anti-inflammatory effects of BAY 11-7082, its ability to induce B-cell lymphoma and leukemic T-cell death and to prevent the recruitment of proteins to sites of DNA damage are exerted via inhibition of components of the ubiquitin system– not by inhibiting NF-κB. BAY11-7082 also inhibits proliferation and promotes apoptosis in breast carcinoma MCF-7 cells by inhibiting phosphorylation of ATP citrate lyase.
storage
+4°C
References
1) Pierce et al. (1997) Novel inhibitor of cytokine-induced IkBα phosphorylation and endothelial cell adhesion molecule expression show anti-inflammatory effects in vivo; J. Biol. Chem., 272 21096 2) Park et al. (2009) 4-hydroxyestradiol induces anchorage-independent growth of human mammary epithelial cells via activation of IkappaB kinase: potential role of reactive oxygen species; Cancer Res., 69 2416 3) Miyamoto et al. (2010) Inhibitor of IkappaB kinase activity, BAY 11-7082, interferes with interferon regulatory factor 7 nuclear translocation and type I interferon production by plasmacytoid dendritic cells; Arthritis Res. Ther., 12 R87 4) Xu et al. (2019) Bay 11-7082 facilitates wound healing by antagonizing mechanical injury – and TNF-α-induced expression of MMPs in posterior cruciate ligament; Connect. Tissue Res. 60 311 5) Kim et al. (2018) TNF-α induces human neural progenitor cell survival after oxygen-glucose deprivation by activating the NFκB pathway; Exp. Mol. Med., 50 14
Properties of BAY 11-7082
| Melting point: | 133-135℃ |
| Boiling point: | 397.6±42.0 °C(Predicted) |
| Density | 1.237±0.06 g/cm3(Predicted) |
| storage temp. | Keep in dark place,Inert atmosphere,2-8°C |
| solubility | DMSO: 25 mg/mL, soluble |
| form | solid |
| color | white |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20° for up to 3 months. |
Safety information for BAY 11-7082
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for BAY 11-7082
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 BAY 11-7082 CAS 19542-67-7View Details
BAY 11-7082 CAS 19542-67-7View Details
19542-67-7 -
 Bay 11-7082 CAS 19542-67-7View Details
Bay 11-7082 CAS 19542-67-7View Details
19542-67-7 -
 BAY 11-7082 CAS 19542-67-7View Details
BAY 11-7082 CAS 19542-67-7View Details
19542-67-7 -
 BAY 11-7082 CASView Details
BAY 11-7082 CASView Details -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1