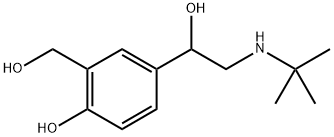
Albuterol sulfate
Synonym(s):α-([t-Butylamino]methyl)-4-hydroxy-m-xylene-α,α′-diol;α1-[(tert-Butylamino)methyl]-4-hydroxy-1,3-benzenedimethanol hemisulfate salt;Albuterol;Albuterol sulfate;Salbutamol hemisulfate salt
- CAS NO.:51022-70-9
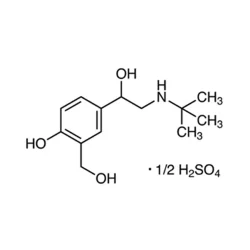
- Empirical Formula: C13H23NO7S
- Molecular Weight: 337.39
- MDL number: MFCD00055200
- EINECS: 256-916-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-16 16:15:04
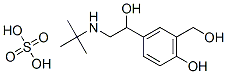
What is Albuterol sulfate?
Description
Albuterol sulfate (Brand name: PROVENTIL® HFA) is the sulfate form of albuterol which is also known as salbutamol. It is a kind of short-acting, selective beta2-adrenergic receptor agonist being capable of opening up the medium and large airways in the lungs. It is mainly used for the treatment of bronchospasm (induced by bronchial asthma or exercise) as well as chronic obstructive pulmonary disease (COPD). Albuterol sulfate takes effects through directly biding to the beta (2)-adrenergic receptors in the lung and causing relaxation of bronchial smooth muscles. This relaxation is mediated by its effect of stimulating cAMP production through activating adenylate cyclase, further inhibiting the phosphorylation of myosin and lowering the intracellular calcium level. Albuterol sulfate can also be used to treat acute hyperkalemia of the blood.
Chemical properties
Albuterol sulfate is a white or practically white powder, freely soluble in water and slightly soluble in ethanol.
The Uses of Albuterol sulfate
Albuterol Sulfate belongs to a class of bronchodilator drugs called β2-adrenoceptor agonists. It is a prescription medicine used to treat symptoms of bronchospasm. Albuterol Sulfate may be used alone or with other medications.
What are the applications of Application
Salbutamol hemisulfate is a β2-AR adrenergic receptor agonist
Definition
ChEBI: Albuterol sulfate is an ethanolamine sulfate salt. It is functionally related to an albuterol.
brand name
Accuneb (Dey); Proair (IVAX); Proventil (Schering); Ventolin (GlaxoSmithKline); Volmax (Muro); Vospire (Odyssey).
General Description
Albuterol belongs to the class of medicines known as bronchodilators. It is also called a short-acting beta-agonist (SABA).
Biological Activity
Non-selective β -adrenergic agonist, more potent at β 2 than β 1 receptors.
Veterinary Drugs and Treatments
Albuterol is used principally in dogs and cats for its effects on bronchial smooth muscle to alleviate bronchospasm or cough. It is also used in horses as a bronchodilator.
Storage
Store at RT
Mode of action
Albuterol Sulfate is the sulfate salt of the short-acting sympathomimetic agent albuterol, a 1:1 racemic mixture of (R)-albuterol and (S)-albuterol with bronchodilator activity. Albuterol stimulates beta2-adrenergic receptors in the lungs, thereby activating the enzyme adenylate cyclase that catalyzes the conversion of ATP to cyclic-3',5'-adenosine monophosphate (cAMP). Increased cAMP concentrations relax bronchial smooth muscle, relieve bronchospasms, and reduce inflammatory cell mediator release, especially from mast cells. To a lesser extent albuterol stimulates beta1-adrenergic receptors, thereby increasing the force and rate of myocardial contraction.
References
https://en.wikipedia.org/wiki/Salbutamol
https://www.drugbank.ca/drugs/DB01001
http://www.rxlist.com/albuterol-sulfate-drug/indications-dosage.htm
Properties of Albuterol sulfate
| Melting point: | 180 °C |
| Flash point: | 250 °C |
| storage temp. | 2-8°C |
| solubility | 1 M NaOH: soluble50 mg/ml, clear to slightly hazy, yellow-green |
| form | neat |
| form | Solid |
| color | White |
| Water Solubility | Soluble in water and 1M sodium hydroxide (50 mg/ml). |
| Merck | 216 |
| InChI | InChI=1S/C13H21NO3.H2O4S/c1-13(2,3)14-7-12(17)9-4-5-11(16)10(6-9)8-15;1-5(2,3)4/h4-6,12,14-17H,7-8H2,1-3H3;(H2,1,2,3,4) |
| CAS DataBase Reference | 51022-70-9(CAS DataBase Reference) |
Safety information for Albuterol sulfate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H317:Sensitisation, Skin H351:Carcinogenicity |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P272:Contaminated work clothing should not be allowed out of the workplace. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Albuterol sulfate
| InChIKey | OVICLFZZVQVVFT-UHFFFAOYSA-N |
| SMILES | CC(C)(NCC(O)C1C=CC(O)=C(CO)C=1)C.S(O)(O)(=O)=O |
Albuterol sulfate manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 Salbutamol sulfate 98%View Details
Salbutamol sulfate 98%View Details -
 Salbutamol sulfate 98%View Details
Salbutamol sulfate 98%View Details -
 Salbutamol sulfate 99%View Details
Salbutamol sulfate 99%View Details
51022-70-9 -
 Salbutamol sulfate 95.00% CAS 51022-70-9View Details
Salbutamol sulfate 95.00% CAS 51022-70-9View Details
51022-70-9 -
 Salbutamol sulfate 98% (HPLC) CAS 51022-70-9View Details
Salbutamol sulfate 98% (HPLC) CAS 51022-70-9View Details
51022-70-9 -
 Salbutamol hemisulfate salt 98% (HPLC) CAS 51022-70-9View Details
Salbutamol hemisulfate salt 98% (HPLC) CAS 51022-70-9View Details
51022-70-9 -
 Salbutamol Sulfate IHRS, 2 mgView Details
Salbutamol Sulfate IHRS, 2 mgView Details
51022-70-9 -
 Salbutamol Sulphate API PowderView Details
Salbutamol Sulphate API PowderView Details
51022-70-9
