BARIUM SULFIDE
- CAS NO.:21109-95-5
- Empirical Formula: BaHS
- Molecular Weight: 170.4
- MDL number: MFCD00014192
- EINECS: 244-214-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:50:40
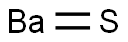
What is BARIUM SULFIDE?
Chemical properties
colourless crystals, or white to grey-brown powder,
Physical properties
Colorless crystalline solid; density 4.25 g/cm3; refractive index 2.155; melts at 1,200°C; soluble in water (decomposes); insoluble in alcohol.
Occurrence
Barium sulfide occurs in the form of black ash, which is a gray to black impure product obtained from high temperature carbonaceous reduction of barite. It is the starting material in the manufacture of most barium compounds including barium chloride and barium carbonate. It is used in luminous paints; for dehairing hides; as a flame retardant; and for generating H2S.
The Uses of BARIUM SULFIDE
Barium sulfide has different characters, so it is used in a variety of ways, such as in electronics, paint pigments, dehairing hides, flame retardant, luminous paints, and generating pure hydrogen sulfide. Barium sulfide can deviate X-rays and helps to give clearly image of the soft tissue. Barium sulfide is a precursor to other barium compounds and also used as a short wavelength emitters for electronic displays.
The Uses of BARIUM SULFIDE
As depilatory; in luminous paints; manufacture of lithopone; vulcanizing rubber, generating H2S.
Production Methods
Barium sulfide is grayish-white solid, formed by heating barium sulfate and carbon, reactive with H2O to form barium hydrosulfide, Ba(SH)2, solution. The latter is also made by saturation of barium hydroxide solution with H2S. Barium polysulfides are formed by boiling barium hydrosulfide with sulfur.
Preparation
Barium sulfide can be
prepared by the direct reaction of the elements, calcined
in an inert atmosphere, at a 1:1.05 molecular ratio:
Ba+S+heat→BaS
Barium sulfide is prepared commercially by heating
barite (BaO) with coal or petroleum coke in a rotary kiln
at 1000°C to 1250°C in an oxygen-free atmosphere. The
product, black ash, is a gray or black powder containing
carbonaceous impurities and unreacted barite. Barium
sulfide is separated from impurities by extraction with
hot water and filtration. Barium sulfide may also be made by high temperature reduction of barium sulfate with methane.
General Description
Barium sulfide (BaS) is an inorganic compound that is used as a precursor in the production of barium compounds. These compounds can be used in a variety of applications, such as ceramics, luminous paints, additives, and flame retardants.
Hazard
Highly toxic by ingestion (see Barium).
Flammability and Explosibility
Non flammable
Safety Profile
A poison. Flammable by spontaneous chemical reaction, air, moisture, or acid fumes may cause it to ignite. For explosion and disaster hazards, see SULFIDES. To fight fire, use CO2, dry chemical. Reacts violently with phosphorus(V) oxide. Mxtures with lead dioxide, potassium chlorate, or potassium nitrite explode when heated. Incompatible with Cl2O, Ca(NO3)2, r(NO3)2, Ca(ClO3)2, Sr(ClO3)2, (ClO3)2. See also BARIUM COMPOUNDS (soluble) and SULFIDES.
Properties of BARIUM SULFIDE
| Melting point: | 1200°C |
| Density | 4.25 g/mL at 15 °C(lit.) |
| form | Powder |
| color | Pale gray to yellow |
| Specific Gravity | 4.25 |
| Water Solubility | Soluble in water but insoluble in alcohol. |
| Sensitive | Moisture Sensitive |
| Merck | 14,995 |
| Stability: | Stable, but decomposes on heating. Incompatible with acids, oxidizing agents, water. |
| CAS DataBase Reference | 21109-95-5(CAS DataBase Reference) |
| NIST Chemistry Reference | Barium sulfide(21109-95-5) |
| EPA Substance Registry System | Barium sulfide (21109-95-5) |
Safety information for BARIUM SULFIDE
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H290:Corrosive to Metals H301:Acute toxicity,oral H314:Skin corrosion/irritation H332:Acute toxicity,inhalation H400:Hazardous to the aquatic environment, acute hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for BARIUM SULFIDE
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 Barium sulfide CAS 21109-95-5View Details
Barium sulfide CAS 21109-95-5View Details
21109-95-5 -
 Barium sulfide CAS 21109-95-5View Details
Barium sulfide CAS 21109-95-5View Details
21109-95-5 -
 Barium sulphide CAS 21109-95-5View Details
Barium sulphide CAS 21109-95-5View Details
21109-95-5 -
 Barium sulphide, 99.9% CAS 21109-95-5View Details
Barium sulphide, 99.9% CAS 21109-95-5View Details
21109-95-5 -
 Barium sulfide CAS 21109-95-5View Details
Barium sulfide CAS 21109-95-5View Details
21109-95-5 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4
