Barium hydroxide octahydrate
Synonym(s):Barium hydroxide octahydrate;Caustic baryta, Barium oxide hydrate octahydrate
- CAS NO.:12230-71-6
- Empirical Formula: BaH4O3
- Molecular Weight: 189.36
- MDL number: MFCD00149152
- EINECS: 602-490-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
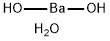
What is Barium hydroxide octahydrate?
Description
Barium hydroxide is available as colourless or white crystals. It is odourless and soluble in water. It is stable under ordinary conditions of use and storage. It is incompatible with acids, oxidisers, and chlorinated rubber. Barium hydroxide is corrosive to metals such as zinc. Barium hydroxide is very alkaline and rapidly absorbs carbon dioxide from air, becoming completely insoluble in water. Barium hydroxide is used in analytical chemistry for the titration of weak acids. Barium hydroxide is used in organic synthesis as a strong base. Barium hydroxide decomposes to barium oxide when heated to 800°C.
Chemical properties
white crystalline powder
The Uses of Barium hydroxide octahydrate
Barium hydroxide octahydrate is extensively used in the manufacturing of barium salts and barium organic compounds; In manufacture of alkali, glass; in synthetic rubber vulcanization, in corrosion inhibitors, pesticides, sugar industry; boiler scale remedy; refining animal and vegetable oils; softening water. Catalyst in organic synthesis.
The Uses of Barium hydroxide octahydrate
In manufacture of alkali, glass; in synthetic rubber vulcanization, in corrosion inhibitors, pesticides, sugar industry; boiler scale remedy; refining animal and vegetable oils; softening water. Catalyst in organic synthesis.
The Uses of Barium hydroxide octahydrate
Used in manufacture of alkali, glass; in synthetic rubber vulcanization, alkalizing agent in water softening, boiler scale remedy, softening water.Barium hydroxide octahydrate is used in the centromeric heterochromatin banding technique. It acts as a precursor to prepare barium titanate, barium sulfate, barium phosphate and barium sulfide. Further, it is used in analytical chemistry for the titration of weak acids especially organic acids. It is involved in the preparation of cyclopentanone, diacetone alcohol and D-Gulonic gamma-lactone. In addition to this, it finds application in ester hydrolysis such as dimethyl hendecanedioate and decarboxylation of amino acids.
What are the applications of Application
Barium hydroxide octahydrate is an agent used in the centromeric heterochromatin banding technnique
What are the applications of Application
Catalyzes the β-elimination of phosphoserine residues.
Resin stabilizer; manufacture of oil and grease additives; raw material of pigment; sulfate removal agent.
Purification Methods
It crystallises from water (1mL/g) and readily absorbs CO2 from air. It effloresces to the monohydrate. It dehydrates to Ba(OH)2 in dry air at 100o. An aqueous solution (baryta water) absorbs CO2 to form a white precipitate of BaCO3.
Properties of Barium hydroxide octahydrate
| Melting point: | 78 °C(lit.) |
| Boiling point: | 780°C |
| Density | 2.18 |
| storage temp. | Store at +5°C to +30°C. |
| solubility | 72g/l |
| form | Solid |
| color | White |
| Specific Gravity | 2.18 |
| PH Range | 12.5 at 50 g/l at 20 °C |
| PH | 14 (H2O, 20℃)(saturated aqueous solution) |
| Odor | Odorless |
| Water Solubility | 56 g/L (15 ºC) |
| Sensitive | Air Sensitive & Hygroscopic |
| Merck | 14,977 |
| Exposure limits | ACGIH: TWA 0.5 mg/m3 NIOSH: IDLH 50 mg/m3; TWA 0.5 mg/m3 |
| Stability: | Stable. |
| CAS DataBase Reference | 12230-71-6(CAS DataBase Reference) |
Safety information for Barium hydroxide octahydrate
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Barium hydroxide octahydrate
Barium hydroxide octahydrate manufacturer
ARRAKIS INDUSTRIES LLP
Buradon Inc.
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran



You may like
-
 Barium hydroxide octahydrate 98%View Details
Barium hydroxide octahydrate 98%View Details
12230-71-6 -
 Barium Hydroxide Octahydrate 98%View Details
Barium Hydroxide Octahydrate 98%View Details -
 Barium hydroxide octahydrate, For ACS analysis CAS 12230-71-6View Details
Barium hydroxide octahydrate, For ACS analysis CAS 12230-71-6View Details
12230-71-6 -
 Barium hydroxide octahydrate, For ACS analysis CAS 12230-71-6View Details
Barium hydroxide octahydrate, For ACS analysis CAS 12230-71-6View Details
12230-71-6 -
 Barium Hydroxide Octahydrate extrapure AR CAS 12230-71-6View Details
Barium Hydroxide Octahydrate extrapure AR CAS 12230-71-6View Details
12230-71-6 -
 Barium hydroxide octahydrate 98%View Details
Barium hydroxide octahydrate 98%View Details -
 Barium Hydroxide Octahydrate pure CAS 12230-71-6View Details
Barium Hydroxide Octahydrate pure CAS 12230-71-6View Details
12230-71-6 -
 Barium hydroxide octahydrate 95% CAS 12230-71-6View Details
Barium hydroxide octahydrate 95% CAS 12230-71-6View Details
12230-71-6
