Aluminum hydroxide
Synonym(s):Aluminium hydroxide;Hydrargillite
- CAS NO.:21645-51-2
- Empirical Formula: AlH3O3
- Molecular Weight: 78
- MDL number: MFCD00003420
- EINECS: 244-492-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-22 18:23:36
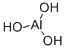
What is Aluminum hydroxide?
Absorption
Approximately 17-30% of the aluminum chloride formed is absorbed.
Description
Aluminum hydroxide, insoluble in water, soluble in dilute mineral acids and alkali hydroxides (4 % suspension in water), white, amorphous powder. For other relevant properties and production, see→Aluminum Oxide, Chap. 1.1. Available as Al(OH)3·nH2O (algedrate) and algedrate hexitol complex.
Chemical properties
Aluminum hydroxide, Al(OH)3, also known as aluminum trihydroxide, aluminum trihydrate, aluminum hydrate, hydrated alumina, and hydrated aluminum oxide, is a white to whitish-yellow water-insoluble powder with a specific gravity of 2.42. Aluminum hydroxide is used as a baseforpigments, as a water repellent in textile coatings, and as an antacid in medicine. Aluminum hydroxide is soluble in hydrochloric or sulfuric acids or in sodiumhydroxide.
Chemical properties
Aluminum hydroxide adjuvant is a white hydrogel that sediments slowly and forms a clear supernatant.
The Uses of Aluminum hydroxide
aluminum hydroxide is an inorganic compound used to make a product less transparent. It is also used by formulators as a humectant, and to soften, smooth, and protect the skin. In addition it helps control product viscosity. often found in facial masks and make-up preparations.
The Uses of Aluminum hydroxide
Mainly used as an Active medicament in an Antacid Formulations, also used in manufacturing of Lake Colors, Inks, glass, effluent treatment, and fire retardants.
The Uses of Aluminum hydroxide
As absorbent, in chromatography, manufacturing of glass, paper, inks, ceramics, lubricants, cosmeticsAluminum hydroxide is used as a desiccant powder, filler in paper, plastics, rubber and cosmetics. It is used as a smoke suppressant and mordant dye. It is also used in drugs as antacid and antihyperphosphatemic. It is also used as a Claus catalyst support for waterproof fabrics. It is an important starting material for the preparation of other aluminum compounds, calcined alumina, aluminum sulfate, polyaluminum chloride, zeolites, sodium aluminate, activated alumina and aluminum nitrate. In addition, it is used as a fire retardant.
What are the applications of Application
Aluminum hydroxide is a catalyst in various chemical processes
Background
Aluminum hydroxide is an inorganic salt used as an antacid. It is a basic compound that acts by neutralizing hydrochloric acid in gastric secretions. Subsequent increases in pH may inhibit the action of pepsin. An increase in bicarbonate ions and prostaglandins may also confer cytoprotective effects.
Indications
For relief of heartburn and acid indigestion.
Production Methods
Aluminum hydroxide adjuvant is prepared by the precipitation of a soluble aluminum salt by an alkali hydroxide, or the precipitation of an alkali aluminate by acid.
Definition
aluminium hydroxide: A white crystalline compound, Al(OH)3; r.d. 2.42-2.52. The compound occurs naturally as the mineral gibbsite (monoclinic). In the laboratory it can be prepared by precipitation from solutions of aluminium salts. Such solutions contain the hexaquoaluminium( III) ion with six water molecules coordinated, [Al(H2O)6]3+. In neutral solution this ionizes:
[Al(H2O)6]3+→H+ + [Al(H2O)5OH]2+
The presence of a weak base such as S2- or CO32- (by bubbling hydrogen sulphide or carbon dioxide through the solution) causes further ionization with precipitation of aluminium hydroxide
[Al(H2O)6]3+(aq) → Al(H2O)3(OH)3(s) + 3H+(aq)
The substance contains coordinated water molecules and is more correctly termed hydrated aluminium hydroxide. In addition, the precipitate has water molecules trapped in it and has a characteristic gelatinous form. The substance is amphoteric. In strong bases the aluminate ion is produced by loss of a further proton:
Al(H2O)3(OH)3(s) + OH-(aq)→
[Al(H2O)2(OH)4]-(aq) + H2O(l)
On heating, the hydroxide transforms to a mixed oxide hydroxide, AlO.OH (rhombic; r.d. 3.01). This substance occurs naturally as diaspore and boehmite. Above 450℃ it transforms to γ-alumina.
In practice various substances can be produced that are mixed crystalline forms of Al(OH)3, AlO.OH, and aluminium oxide (Al2O3) with water molecules. These are known as hydrated alumina. Heating the hydrated hydroxide causes loss of water, and produces various activated aluminas, which differ in porosity, number of remaining -OH groups, and particle size. These are used as catalysts (particularly for organic dehydration reactions), as catalyst supports, and in chromatography. Gelatinous freshly precipitated aluminium hydroxide was formerly widely used as a mordant for dyeing and calico printing because of its ability to form insoluble coloured lakes with vegetable dyes.
brand name
Amphojel (Wyeth-Ayerst); Dialume (Rhone-Poulenc Rorer).
Flammability and Explosibility
Non flammable
Pharmaceutical Applications
Aluminum hydroxide adjuvant is used in parenteral human and veterinary vaccines.It activates Th2 immune responses, including IgG and IgE antibody responses. It is also used for the isolation of certain serum components such as blood clotting factors.
Pharmaceutical Applications
Aluminium hydroxide (Al(OH)3) has several medical applications. It is used as an antacid for treating
heartburn as well as acid indigestion (reflux oesophagitis). It is also known to have healing properties of peptic ulcers. In patients suffering from kidney failure, who show elevated serum phosphate levels (hyperphosphataemia),
Al(OH)3 is used as a phosphate binder.
Al(OH)3 is an amphoteric compound , which means it can react as a base or as an
acid. In its application as an anti-acid, Al(OH)3 reacts with any excess stomach acid (mainly HCl) with the
formation of AlCl3 and water .
Al(OH)3 + 3HCl → AlCl3 + 3H2O
Al(OH)3 is known to cause constipation, so formulations of anti-acids often include a combination with
Mg2+ antacids. Usually, oral antifoaming agents, such as simethicone, are added in order to reduce bloating and discomfort/pain.
Pharmacokinetics
Gastric-peptic disease occurs as a result of an imbalance between protective factors, such as mucus, bicarbonate, and prostaglandin secretion, and aggressive factors, such as hydrochloric acid, pepsin, and Helicobacter pylori (H. pylori). Antacids work by restoring acid-base balance, attenuating the pepsin activity and increasing bicarbonate and prostaglandin secretion.
Clinical Use
Phosphate binding agent
Antacid
Safety Profile
Poison by intraperitoneal route. Human systemic effects by ingestion: fever, osteomalacia, and gastrointestinal effects. When coprecipitated with bismuth hydroxide and reduced by H2, it is violently flammable in air. Incompatible with chlorinated rubber.
Safety
Aluminum hydroxide adjuvant is intended for use in parenteral vaccines and is generally regarded as nontoxic. It may cause mild irritation, dryness, and dermatitis on skin contact. On eye contact, aluminum hydroxide adjuvant may also cause redness, conjunctivitis, and short-term mild irritation. Ingestion of large amounts may cause gastrointestinal irritation with nausea, vomiting, and constipation. Inhalation of the dried product may cause respiratory irritation and cough. Type I hypersensitivity reactions following parenteral administration have been reported.
Veterinary Drugs and Treatments
Orally administered aluminum hydroxide is used to reduce hyperphosphatemia in patients with renal failure.
Drug interactions
Potentially hazardous interactions with other drugs
Cytotoxics: concentration of dasatinib and erlotinib
possibly reduced - give at least 4 hours before or 2
hours after erlotinib.
Metabolism
Not metabolized.
Metabolism
Aluminum hydroxide or oxide is slowly solubilised in the stomach and reacts with hydrochloric acid to form aluminium chloride and water. In addition to forming aluminium chloride, dihydroxyaluminium sodium carbonate and aluminium carbonate form carbon dioxide, and aluminium phosphate forms phosphoric acid. About 17-30% of the aluminium chloride formed is absorbed and is rapidly excreted by the kidneys in patients with normal renal function. Aluminium-containing antacids also combine with dietary phosphate in the intestine forming insoluble, nonabsorbable aluminium phosphate which is excreted in the faeces.
Storage
Aluminum hydroxide adjuvant is stable for at least 2 years when stored at 4–308℃ in well-sealed inert containers. It must not be allowed to freeze as the hydrated colloid structure will be irreversibly damaged.
Incompatibilities
When exposed to phosphate, carbonate, sulfate, or borate anions, the point of zero charge for aluminum hydroxide adjuvant decreases.
Regulatory Status
GRAS listed. Accepted for use in human and veterinary parenteral vaccines in Europe and the USA. The limits for use in human vaccines are 0.85 mg aluminum/dose (FDA) and 1.25 mg aluminum/ dose (WHO). There are no established limits for use in veterinary vaccines. Reported in the EPA TSCA Inventory.
Properties of Aluminum hydroxide
| Melting point: | 300℃ |
| Boiling point: | 2980℃[at 101 325 Pa] |
| Density | 2.42 g/cm3 at 20 °C |
| vapor pressure | <0.1 hPa (20 °C) |
| refractive index | Average refractive index: 1.57-1.59 |
| storage temp. | Store at +5°C to +30°C. |
| solubility | 0.0015g/l |
| form | colloidal suspension |
| color | White |
| Specific Gravity | 2.42 |
| Odor | Odorless |
| PH Range | >7 |
| PH | 8-9 (100g/l, H2O, 20℃)(slurry) |
| Water Solubility | insoluble |
| Crystal Structure | Monoclinic |
| Merck | 14,342 |
| Solubility Product Constant (Ksp) | pKsp: 32.89 |
| Exposure limits | ACGIH: TWA 1 mg/m3 |
| Dielectric constant | 2.2(Ambient) |
| Stability: | Stable. Incompatible with strong bases. |
| CAS DataBase Reference | 21645-51-2(CAS DataBase Reference) |
| EPA Substance Registry System | Aluminum hydroxide (21645-51-2) |
Safety information for Aluminum hydroxide
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for Aluminum hydroxide
Aluminum hydroxide manufacturer
JSK Chemicals
NRS Chemicals LLP
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 ALUMINIUM HYDROXIDE GEL 99%View Details
ALUMINIUM HYDROXIDE GEL 99%View Details -
 ALUMINUM HYDROXIDE 98%View Details
ALUMINUM HYDROXIDE 98%View Details -
 Aluminum hydroxide CAS 21645-51-2View Details
Aluminum hydroxide CAS 21645-51-2View Details
21645-51-2 -
 Aluminum hydroxide CAS 21645-51-2View Details
Aluminum hydroxide CAS 21645-51-2View Details
21645-51-2 -
 Aluminum hydroxide CAS 21645-51-2View Details
Aluminum hydroxide CAS 21645-51-2View Details
21645-51-2 -
 Dried Hydroxide, Aluminium Gel CAS 21645-51-2View Details
Dried Hydroxide, Aluminium Gel CAS 21645-51-2View Details
21645-51-2 -
 Aluminum Hydroxide Powder, Grade Standard: INDUSTRIALView Details
Aluminum Hydroxide Powder, Grade Standard: INDUSTRIALView Details
21645-51-2 -
 Aluminium Hydroxide, Packaging Type: Hdpe Bag, Packaging Size: 25 KgsView Details
Aluminium Hydroxide, Packaging Type: Hdpe Bag, Packaging Size: 25 KgsView Details
21645-51-2
