Barium hydroxide
Synonym(s):Barium dihydroxide
- CAS NO.:17194-00-2
- Empirical Formula: BaH2O2
- Molecular Weight: 171.34
- MDL number: MFCD00003443
- EINECS: 241-234-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 15:25:55
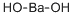
What is Barium hydroxide ?
Description
The octahydrate of barium hydroxide is freely soluble in water and MeOH, whereas the monohydrate is only slightly soluble in water. For this reason the octahydrate is the most common form.
Chemical properties
Barium hydroxide,Ba(OH)2·8H20, is a white crystalline powder, colorless solid composed of monoclinic crystals. It has a melting point of 78°C and is soluble in water and insoluble in acetone. It is used in the fusion of silicates and fat saponification.
Physical properties
Monohydrate, Ba(OH)2?H2O is a white powder; density 3.743 g/cm3; slightly soluble in water; soluble in dilute mineral acids. Octahydrate, Ba(OH)2?8H2O is a colorless monoclinic crystal; density 2.18 g/cm3 at 16°C; refractive index 1.50; melts at 78°C; vapor pressure 227 torr; loses seven molecules of water of crystallization when its solution is boiled in the absence of atmospheric CO2 forming solid monohydrate; further heating produces anhydrous Ba(OH)2 melting at 407°C; readily dissolves in water (3.76 g/100 g at 20°C and 11.7 g/100 g at 50°C); aqueous solution highly alkaline; also soluble in methanol; slightly soluble in ethanol; insoluble in acetone.
The Uses of Barium hydroxide

To a solution of KOH (10 g, 178 mmol) in H2O (15 mL) and EtOH (15 mL) at RT was added the SM (3.92 g, 42 mmol) over 10 min. The reaction mixture was stirred at 90 C for 3.5 h, after which time it was concentrated in vacuo. The resulting residue was dissolved in H2O (10 mL) and acidified at 0 C with 6M HCl to pH 1. The mixture was extracted with DCM. The org layer was dried (MgSO4) and concentrated to provide the product as a colorless oil which was taken forward without further purification. [4.65 g, 98%]
Preparation
Barium hydroxide is made by dissolving barium oxide in hot water. The octahydrate, Ba(OH)2?8H2O, crystallizes upon cooling. It also is prepared by precipitation with caustic soda from an aqueous solution of barium sulfide:
BaS + 2NaOH → Ba(OH)2 + Na2S.
Hazard
An acute poison; toxic symptoms are similar to other soluble salts of barium (see Barium).
Flammability and Explosibility
Not classified
Safety Profile
A poison by ingestion andintraperitoneal routes. Incompatible with chlorinated rubber.
The Uses of Barium hydroxide
Barium hydroxide is defined as a chemical compound with the formula Ba(OH)2, which can exist in a hydrated form as barium hydroxide octahydrate (Ba(OH)2·8H2O) and is studied for its potential as a thermal energy storage material due to its stable thermal properties after multiple cycles.
Production
Barium hydroxide can be prepared by dissolving barium oxide (BaO) in water.
Properties of Barium hydroxide
| Melting point: | >300 °C(lit.) |
| Boiling point: | decomposes at 1032 (calculated) [KNA91] |
| Density | 2.2 g/mL at 25 °C(lit.) |
| solubility | Aqueous Acid (Slightly), Water (Slightly) |
| form | Solid |
| appearance | White solid (monohydrate) |
| color | Clear |
| Odor | no odor |
| PH | 11.27(1 mM solution);12.22(10 mM solution);13.08(100 mM solution) |
| Water Solubility | Soluble in water. |
| Sensitive | Air Sensitive & Hygroscopic |
| Merck | 14,977 |
| Exposure limits | ACGIH: TWA 0.5 mg/m3 NIOSH: IDLH 50 mg/m3; TWA 0.5 mg/m3 |
| Stability: | Stable. Incompatible with acids, carbon dioxide, moisture. |
| CAS DataBase Reference | 17194-00-2(CAS DataBase Reference) |
| NIST Chemistry Reference | Barium dihydroxide(17194-00-2) |
| EPA Substance Registry System | Barium hydroxide (Ba(OH)2) (17194-00-2) |
Safety information for Barium hydroxide
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Barium hydroxide
| InChIKey | RQPZNWPYLFFXCP-UHFFFAOYSA-L |
Barium hydroxide manufacturer
Jaiswal S Cyber Shop
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
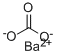



You may like
-
 Barium hydroxide 98%View Details
Barium hydroxide 98%View Details -
 Barium hydroxide, anhydrous, anhydrous CAS 17194-00-2View Details
Barium hydroxide, anhydrous, anhydrous CAS 17194-00-2View Details
17194-00-2 -
 Barium hydroxide, anhydrous CAS 17194-00-2View Details
Barium hydroxide, anhydrous CAS 17194-00-2View Details
17194-00-2 -
 Barium hydroxide, anhydrous, anhydrous CAS 17194-00-2View Details
Barium hydroxide, anhydrous, anhydrous CAS 17194-00-2View Details
17194-00-2 -
 barium hydroxide CASView Details
barium hydroxide CASView Details -
 BARIUM HYDROXIDEView Details
BARIUM HYDROXIDEView Details
17194-00-2 -
 Barium Hydroxide, Packaging Size: 25 KgView Details
Barium Hydroxide, Packaging Size: 25 KgView Details
17194-00-2 -
 Barium Hydroxide Chemical, 50kg bagView Details
Barium Hydroxide Chemical, 50kg bagView Details
17194-00-2
