Cesium
- CAS NO.:7440-46-2
- Empirical Formula: Cs
- Molecular Weight: 132.91
- MDL number: MFCD00134037
- EINECS: 231-155-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-10 11:56:18
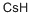
What is Cesium?
Description
Cesium was discovered in 1860 by Robert Bunsen and Gustav Kirchoff. It is used in the most accurate atomic clocks. Cesium melts at 28.41°C (just below body temperature) and occurs in Earth’s crust at 2.6 ppm. Cesium is the rarest of the naturally occurring alkali metals as the isotope 133Cs. Its compounds are correspondingly rare. Granites contain about 1 ppm cesium and sedimentary rocks contain approximately 4 ppm cesium. The most common commercial source of cesium is pollucite, which contains between 5 and 32% cesium oxide. Radioactive forms of cesium (134Cs and 137Cs) can also be found in the environment. They are produced during nuclear fission, and are used in cancer treatment.
Chemical properties
Cesium is silvery gold, soft ductile metal. It is the most electropositive and alkaline element. Cesium, gallium, and mercury are the only three metals that are liquid at or around room temperature. Cesium reacts explosively with cold water, and reacts with ice at temperatures above -116℃. Cesium hydroxide is a strong base and attacks glass and reacts with halogens to form a fluoride, chloride, bromide, and iodide. Cesium metal oxidizes rapidly when exposed to air and can form the dangerous superoxide on its surface. Most cesium compounds are water soluble.

Physical properties
Like the other alkali metals, cesium is a soft-solid silvery metal, but much softer than theothers. It is the least electronegative and most reactive of the Earth metals. Cesium has anoxidation state of +1, and because its atoms are larger than Li, Na, and K atoms, it readilygives up its single outer valence electron. The single electron in the P shell is weakly attachedto its nucleus and thus available to combine with many other elements. It is much too reactiveto be found in its metallic state on Earth.Cs has a melting point of 29°C, which is lower than the body temperature of humans(37°C), and thus a chunk of cesium will melt in a person’s hand with disastrous results. Sinceit reacts with moisture on skin as well as with the air to release hydrogen, it will burn vigorously through the palm of one’s hand.Cesium’s boiling point is 669.3°C and its density is 1.837 g/cm3. Mercury is the only metalwith a lower melting point than cesium. It is extremely dangerous when exposed to air, water,and organic compounds or to sulfur, phosphorus, and any other electronegative elements. Itmust be stored in a glass container containing an inert atmosphere or in kerosene.Cesium reacts with water in ways similar to potassium and rubidium metals. In additionto hydrogen, it forms what is known as superoxides, which are identified with the generalformula CsO2. When these superoxides react with carbon dioxide, they release oxygen gas,which makes this reaction useful for self-contained breathing devices used by firemen andothers exposed to toxic environments.
Isotopes
Cs-133 is the only stable isotope of cesium, and it makes up all of the naturallyoccurring cesium found in the Earth’s crust. In addition to Cs-133 there are about 36radioactive isotopes of Cs, most of which are artificially formed in nuclear reactors. Allare produced in small numbers of atoms with relatively short half-lives. The range of Csisotopes is from Cs-113 (amu = 112.94451) to Cs-148 (amu = 147.94900). Most ofthese radioisotopes produce beta radiation as they rapidly decay, with the exception ofCs-135, which has a half-life of 3×106yr, which makes it a useful research tool. Cs-137,with a half-life of 33 years, produces both beta and gamma radiation.
Origin of Name
In 1860 Gustav Kirchhoff and Robert Bunsen named the element “Cesium,” using the Latin word caesius, which means bluish-gray.
Occurrence
The stable form of Cs-133 is the 48th most abundant element on Earth, but because it isso reactive, it is always in compound form. The Earth’s crust contains only about 7 ppm ofCs-133. Like the other alkali metals, it is found in mixtures of complex minerals. Its mainsource is the mineral pollucite (CsAlSi2O6). It is also found in lepidolite, a potassium ore.Pollucite is found in Maine, South Dakota, Manitoba, and Elba and primarily in Rhodesia,South Africa.One problem in refining cesium is that it is usually found along with rubidium; therefore,the two elements must be separated after they are extracted from their sources. The mainprocess to produce cesium is to finely grind its ores and then heat the mix to about 600°Calong with liquid sodium, which produces an alloy of Na, Cs, and Ru, which are separatedby fractional distillation. Cesium can also be produced by the thermochemical reduction of amixture of cesium chloride (CsCl) and calcium (Cs).
History
Cesium was discovered spectroscopically by Bunsen and Kirchhoff in 1860 in mineral water from Durkheim. Cesium, an alkali metal, occurs in lepidolite, pollucite (a hydrated silicate of aluminum and cesium), and in other sources. One of the world’s richest sources of cesium is located at Bernic Lake, Manitoba. The deposits are estimated to contain 300,000 tons of pollucite, averaging 20% cesium. It can be isolated by electrolysis of the fused cyanide and by a number of other methods. Very pure, gas-free cesium can be prepared by thermal decomposition of cesium azide. The metal is characterized by a spectrum containing two bright lines in the blue along with several others in the red, yellow, and green. It is silvery white, soft, and ductile. It is the most electropositive and most alkaline element. Cesium, gallium, and mercury are the only three metals that are liquid at room temperature. Cesium reacts explosively with cold water, and reacts with ice at temperatures above –116°C. Cesium hydroxide, the strongest base known, attacks glass. Because of its great affinity for oxygen the metal is used as a “getter” in electron tubes. It is also used in photoelectric cells, as well as a catalyst in the hydrogenation of certain organic compounds. The metal has recently found application in ion propulsion systems. Cesium is used in atomic clocks, which are accurate to 5 s in 300 years. A second of time is now defined as being the duration of 9,192,631,770 periods of the radiation corresponding to the transition between the two hyper-fine levels of the ground state of the cesium-133 atom. Its chief compounds are the chloride and the nitrate. Cesium has 52 isotopes and isomers with masses ranging from 112 to 148. The present price of cesium is about $50/g (99.98%) sealed in a glass ampoule.
Characteristics
Cesium is located between rubidium and francium in group 1 of the periodic table. It isthe heaviest of the stable alkali metals and has the lowest melting point. It is also the mostreactive of the alkali metals.Cesium will decompose water, producing hydrogen, which will burn as it is liberated fromH2O. Cesium is extremely dangerous to handle and will burn spontaneously or explode whenexposed to air, water, and many organic compounds.
The Uses of Cesium
Cesium is used in photovoltaic cells, vacuum tubes, scintillation counters, and atomic clocks.
The Uses of Cesium
In photoelectric cells, as a "getter" in vacuum tubes; in photoemitter devices, scintillation counters. Adsorbent in CO2 purifn; scavenger of gases and impurities in metallurgy. For doping catalysts. For construction and operation of one type of atomic clock based on the vibrational frequency (9,192.76 megacycles/sec) of 133Cs. 137Cs in process control instruments, sewage and sludge sterilization.
The Uses of Cesium
Because of some of its longer-lived isotopes, cesium has become valuable for its ability toproduce a steady stream of beta particles (β) as electrons.Light is strong enough to “knock off” electrons from cesium, which makes this phenomenon useful as a coating for photoelectric cells and electric eye devices. Cesium iodide (CsI)is used in scintillation counters (Geiger counters) to measure levels of external radiation. It isalso useful as a “getter” to remove air molecules remaining in vacuum tubes.In 1960 the International Committee of Weights and Measures selected radioactive cesium-137 (with a half-life of about 33 years) as the standard for measuring time. They equated thesecond with the radiation emitted by a Cs-137 atom that is excited by a small energy source.Thus, the second is now defined as 9,192,631,770 vibrations of the radiation emitted by anatom of Cs-137. There are about 200 atomic clocks around the world that collaborate theirefforts to maintain this extremely accurate clock that never needs winding or batteries.62 | The History and Use of Our Earth’s Chemical ElementsCesium is used as a hydrogenation catalyst to enhance and assist the reaction in the conversion of liquid oils to solids forms (e.g., in the production of margarine).In a molten state, it is used as a heat-transfer fluid in plants generating electric power.Cesium is used experimentally as a plasma to produce a source of ions to power outer spacevehicles using ion engines.Cesium is used in military infrared devices and signal lamps as well as in other opticaldevices.Cesium is used as a chemical reagent and reducing agent in industry and the laboratory. Itcan also be used as an antidote for arsenic poisoning.
Definition
A soft golden highly reactive low-melting element of the alkali-metal group. It is found in several silicate minerals, including pollucite (CsAlSi2O6). The metal oxidizes in air and reacts violently with water. Cesium is used in photocells, as a catalyst, and in the cesium atomic clock. The radioactive isotopes 134Cs (half life 2.065 years) and 137Cs (half life 30.3 years) are produced in nuclear reactors and are potentially dangerous atmospheric pollutants.
Definition
caesium: Symbol Cs. A soft silvery white metallic element belonging to group 1 (formerly IA) of the periodictable; a.n. 55; r.a.m. 132.905; r.d.1.88; m.p. 28.4°C; b.p. 678°C. It occursin small amounts in a numberof minerals, the main source beingcarnallite (KCl.MgCl2.6H2O). It is obtainedby electrolysis of molten caesiumcyanide. The natural isotope iscaesium–133. There are 15 otherradioactive isotopes. Caesium–137(half-life 33 years) is used as a gammasource. As the heaviest alkali metal,caesium has the lowest ionization potentialof all elements, hence its usein photoelectric cells, etc.
Preparation
Although Cesium metals have been prepared by fused salt electrolysis, the highly reactive nature of the metals complicates the collection step and favors the use of other preparative methods where the metals can be removed in vapor form from the reaction mixture. The oxides, hydroxides, carbonates, halides, sulphates, chromates and nitrates of Cesium have been reduced to the metals by strong reducing metals such as sodium, calcium, magnesium, barium, iron, zirconium, aluminum or silicon at moderately high temperatures. The preferred method, however, involves the reduction of the anhydrous metal chlorides with calcium metal under vacuum. Anhydrous cesium chloride is mixed with a large excess of calcium chips and heated under vacuum at 700-800°C. As the chloride is reduced, metal vapors issue from the reaction mixture and are led under the vacuum to a cooler portion of the vessel where they condense and drop into a collection vessel.
General Description
A soft metallic solid. Melts at 85°F. Causes burns to skin and eyes.
Air & Water Reactions
Highly flammable. Cesium is spontaneously flammable in air at room temperature, if the surface is clean [Merck 11th ed. 1989]. Reacts with water to generate enough heat to ignite the hydrogen produced during the reaction, and to generate caustic Cesium hydroxide [Mellor 2 419 1946-47].
Reactivity Profile
Cesium METAL reacts violently with oxidizing agents, even weaker ones. Reacts with boron trifluoride with incandescence when heated [Merck 11th ed. 1989]. Reacts explosively with maleic anhydride [Chem Safety Data Sheet SD-88 1962; Chem. Haz. Info. Series C-71 1960]. Burns in chlorine with a luminous flame [Mellor 2 Supp. 1:380 1956]. Reacts violently with most acids. Reacts violently with fluorine, chlorine, bromine and iodine. Reacts with incandescence with sulfur and phosphorus. Burns vigorously in air.
Hazard
Although cesium has many of the properties and characteristics of the other alkali metals,because of the large size of its atoms, cesium metal is much more reactive and dangerousto handle. Special precautions need to be taken to keep it away from air, water, and organicsubstances with which it can vigorously react. Its use should be restricted to laboratories andindustries capable of using it safely.
Cesium-137, with a half-life of about 30 years, produces dangerous radiation and can causeradiation poisoning if mishandled. It is used to sterilize wheat, potatoes, and other foods toprotect them from insect damage and rotting. It is also used to kill bacteria in the treatmentof sewage sludge.
Health Hazard
Inhalation or contact with vapors, substance or decomposition products may cause severe injury or death. May produce corrosive solutions on contact with water. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control may cause pollution.
Fire Hazard
Produce flammable gases on contact with water. May ignite on contact with water or moist air. Some react vigorously or explosively on contact with water. May be ignited by heat, sparks or flames. May re-ignite after fire is extinguished. Some are transported in highly flammable liquids. Runoff may create fire or explosion hazard.
Industrial uses
A chemical element, cesium (symbol Cs) is theheaviest of the alkali metals in group I. It is asoft, light, very low melting temperature metal.It is the most reactive of the alkali metals andindeed is the most electropositive and the mostreactive of all the elements.
Cesium oxidizes easily in the air, ignites atordinary temperatures, and decomposes waterwith explosive violence. It can be contained invacuum, inert gas, or anhydrous liquid hydrocarbonsprotected from O2 and air. The specificgravity is 1.903, melting point 28.5°C, and boilingpoint 670°C. It is used in low-voltage tubesto scavenge the last traces of air. It is usuallymarketed in the form of its compounds such ascesium nitrate, CsNO3, cesium fluoride, CsF, orcesium carbonate, Cs2CO3. In the form ofcesium chloride, CsCl, it is used on the filamentsof radio tubes to increase sensitivity. Itinteracts with the thorium of the filament toproduce positive tons. In photoelectric cellsCsCl is used for a photosensitive deposit on thecathode, since cesium releases its outer electronunder the action of ordinary light, and its colorsensitivity is higher than that of other alkali metals. The high-voltage rectifying tube forchanging AC to DC has cesium metal coatedon the nickel cathode, and has cesium vapor forcurrent carrying. The cesium metal gives off acopious flow of electrons and is continuouslyrenewed from the vapor. Cesium vapor is alsoused in the infrared signaling lamp as it producesinfrared waves without visible light. Cesiumsalts have been used medicinally as antishockagents after administration of arsenic drugs.Cesium metal is generaly made by thermochemicalprocesses. The carbonate can bereduced by metallic magnesium, or the chloridecan be reduced by CaC. Metallic cesium volatilizesfrom the reaction mixture and is collectedby cooling the vapor.
Safety Profile
Moderately toxic by intraperitoneal route. Cesium is quite similar to potassium in its elemental state. It has been shown, however, to have pronounced physiological action in experimentation with animals. Hyper-irritability, including marked spasms, has been shown to follow the administration of cesium in amounts equal to the potassium content of the diet. It has been found that replacing the potassium in the diet of rats with cesium caused death after 10-17 days. Ignites spontaneously in air. Violent reaction with water, moisture, or steam releases hydrogen gas whch explodes. Violent reaction with acids, halogens, and other oxidizing materials. Incandescent reaction with nonmetals (e.g., sulfur, phosphorus). See also SODIUM.
Environmental Fate
Stable cesium was shown to affect various central nervous
system functions, mainly involving displacing potassium, with
which it competes for transport through the potassium
channel, and it can also activate sodium pump and subsequent
transport into the cell across membranes. Thus, this resulted in
potassium deficiency.
Radioactive isotopes of cesium, such as 134Cs and 137Cs, are
a greater health concern than stable cesium. These radioactive
isotopes of cesium are formed during nuclear fission. Both
134Cs and 137Cs emit beta and gamma radiations. Beta radiation
travels short distances and can penetrate the skin and
superficial body tissues, whereas gamma radiation can travel
great distances and penetrate the entire body. Both beta and
gamma radiations may induce tissue damage and disruption of
cellular function.
Toxicity evaluation
Naturally occurring cesium can enter the environment mostly from the erosion and weathering of rocks and minerals. The production and use of cesium compounds may also result in their release to the environment through various waste streams. However, there are relatively few commercial uses for cesium compounds, such as cesium radioactive isotopes (134Cs and 137Cs), and they have been released into the environment by human activities such as the atmospheric testing of nuclear weapons (1945–80) and leakages at nuclear power plants. Cesium compounds can travel long distances in the air before being brought back to the earth by rainfall and gravitational settling. If released to water, cesium compounds are deposited on land and water via wet and dry deposition. These deposited-cesium particles may be resuspended into the atmosphere from soil and dust. If released to soil, cesium compounds have low mobility and do not migrate below 40 cm in depth. The majority of cesium ions are retained in the upper 20 cm of the soil surface. Clay and zeolite minerals strongly bind cesium cations irreversibly. Soils rich in organic matter also adsorb cesium ions. However, cesium compounds are readily exchangeable and highly available for plant uptake in these soils. If released into water, cesium compounds are very water soluble and exist primarily as cesium cations. Because most cesium compounds are ionic, they will not volatilize from water surfaces. Most cesium compounds released to water adsorb to suspended solids in the water column and ultimately they are deposited in sediments. Cesium compounds bioconcentrate and have been shown to bioaccumulate in both terrestrial and aquatic food chains. The half-life of 134Cs is ~2 years and that of 137Cs is~30 years.
Properties of Cesium
| Melting point: | 28.5 °C (lit.) |
| Boiling point: | 705 °C (lit.) |
| Density | 1.873 g/mL at 25 °C (lit.) |
| vapor pressure | 1 mm Hg ( 279 °C) |
| storage temp. | 2-8°C |
| solubility | H2O: soluble |
| form | ingot |
| color | Silver |
| Specific Gravity | 1.892 |
| Resistivity | 19 μΩ-cm, 0°C |
| Water Solubility | reacts with H2O to evolve H2; soluble liquid NH3 [MER06] |
| Sensitive | moisture sensitive |
| Merck | 13,2018 |
| Exposure limits | ACGIH: TWA 2 ppm; STEL 4 ppm OSHA: TWA 2 ppm(5 mg/m3) NIOSH: IDLH 25 ppm; TWA 2 ppm(5 mg/m3); STEL 4 ppm(10 mg/m3) |
| Stability: | Flammable solid; highly flammable in powder form. Moisture-sensitive. Incompatible with chlorine, phosphorus, water. |
| CAS DataBase Reference | 7440-46-2(CAS DataBase Reference) |
| EPA Substance Registry System | Cesium (7440-46-2) |
Safety information for Cesium
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H260:Substances And Mixtures Which, In Contact With Water,Emit Flammable Gases H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P223:Keep away from any possible contact with water, because of violent reaction and possible flash fire. P280:Wear protective gloves/protective clothing/eye protection/face protection. P231+P232:Handle under inert gas. Protect from moisture. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P370+P378:In case of fire: Use … for extinction. P422:Store contents under … |
Computed Descriptors for Cesium
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 Cesium CAS 7440-46-2View Details
Cesium CAS 7440-46-2View Details
7440-46-2 -
 Cesium CAS 7440-46-2View Details
Cesium CAS 7440-46-2View Details
7440-46-2 -
 Cesium CAS 7440-46-2View Details
Cesium CAS 7440-46-2View Details
7440-46-2 -
 Cesium CAS 7440-46-2View Details
Cesium CAS 7440-46-2View Details
7440-46-2 -
 Cesium CAS 7440-46-2View Details
Cesium CAS 7440-46-2View Details
7440-46-2 -
 Cesium, (metals basis) CAS 7440-46-2View Details
Cesium, (metals basis) CAS 7440-46-2View Details
7440-46-2 -
 Cesium CAS 7440-46-2View Details
Cesium CAS 7440-46-2View Details
7440-46-2 -
 Cesium CASView Details
Cesium CASView Details
