Atracurium besylate
- CAS NO.:64228-81-5
- Empirical Formula: C65H82N2O18S2
- Molecular Weight: 1243.48
- MDL number: MFCD00797403
- EINECS: 264-743-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-20 15:18:15
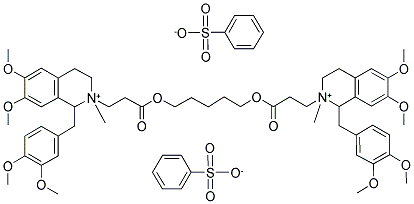
What is Atracurium besylate ?
Toxicity
Excessive doses can be expected to produce enhanced pharmacological effects. Overdosage may increase the risk of histamine release and cardiovascular effects, especially hypotension.
Chemical properties
White to yellowish-white powder, slightly hygroscopic
Originator
Tracrium,Burroughs-Wellcome,US,1983
The Uses of Atracurium besylate
A neuromuscular blocking agent
Indications
For use, as an adjunct to general anesthesia, to facilitate endotracheal intubation and to provide skeletal muscle relaxation during surgery or mechanical ventilation.
What are the applications of Application
Atracurium Besylate is a neuromuscular blocking agent
Background
A non-depolarizing neuromuscular blocking agent with short duration of action. Its lack of significant cardiovascular effects and its lack of dependence on good kidney function for elimination provide clinical advantage over alternate non-depolarizing neuromuscular blocking agents.
Definition
ChEBI: The bisbenzenesulfonate salt of atracurium.
Manufacturing Process
Acryloyl chloride (0.2 mol) in dry benzene (60 ml) was added over 0.5 hour
with mechanical stirring to pentane-1,5-diol (0.1 mol), triethylamine (0.2 mol)
and pyrogallol (0.1 g) in dry benzene (100 ml). Further dry benzene (ca 100
ml) was added followed by triethylamine (10 ml), and the mixture stirred at
50°C for 0.5 hour. The triethylamine hydrochloride was filtered off and the
solvent removed in vacuum to leave a yellow oil which was distilled in the
presence of a trace of p-methoxyphenol, excluding light, to give 1,5-
pentamethylenediacrylate (12.9 g, 61%, BP 90° to 95°C/0.01 mm Hg).
A solution of tetrahydropapaverine (4.43 g) and 1,5-pentamethylene
diacrylate (1.30 g) in dry benzene (15 ml) was stirred under reflux for 48
hours excluding light. The solvent was removed in vacuum and the residual
pale red oil dissolved in chloroform (10 ml). Addition of ether (ca 400 ml),
followed by saturated ethereal oxalic acid solution (ca 500 ml) gave a
flocculent white precipitate, which was filtered off, washed with ether and dried. Crystallization (twice) from ethanol gave N,N'-4,10-dioxa-3,11-
dioxotridecylene-1,13-bis-tetrahydropapaverine dioxalate as a white powder
(3.5 g, 51%, MP 117° to 121°C).
The free base, N,N'-14,10-dioxa-3,11-dioxotridecylene-1,13-bistetrahydropapaverine,
was obtained by basifying an aqueous solution of the
dioxalate with sodium bicarbonate solution, followed by extraction with
toluene and evaporation of the solvent, to give a colorless viscous oil.
Scrupulously dried base (0.5 g) in spectroscopically pure acetonitrile (8 ml)
was treated with methyl benzene sulfonate at room temperature for 22 hours.
The filtered reaction mixture was added dropwise to mechanically stirred,
filtered, dry ether (ca 450 ml). The flocculent white precipitate was filtered off,
washed with dry ether, and dried in vacuum over P2O5 at 50°C to yield the
product, an off-white powder melting at 85° to 90°C.
In practice it is usually used as dibenzenesulfonate.
brand name
Tracrium (Hospira).
Therapeutic Function
Neuromuscular blocker
Biological Functions
Atracurium besylate (Tracrium) is a benzylisoquinolinium compound like d-tubocurarine. Its actions are similar to those of d-tubocurarine, but its duration of action is shorter (45 minutes) because of spontaneous degradation of the molecule (Hofmann elimination). Because of this, atracurium is useful in patients with low or atypical plasma cholinesterase and in patients with renal or hepatic impairment.
General Description
Atracurium besylate, 2-(2-carboxyethyl)-1,2,3,4-tetrahydro-6,7-dimethoxy-2-methyl-1-veratrylisoquinolinium benzenesulfonate pentamethyleneester (Tracrium), is a nondepolarizing neuromuscular blockingagent that is approximately 2.5 times more potent thand-tubocurarine. Its duration of action (half-life, 0.33 hours)is much shorter than that of d-tubocurarine.
Biochem/physiol Actions
Nicotinic acetylcholine receptor antagonist.
Pharmacokinetics
Atracurium is a nondepolarizing skeletal muscle relaxant. Atracurium can be used most advantageously if muscle twitch response to peripheral nerve stimulation is monitored to assess degree of muscle relaxation. The duration of neuromuscular block produced by Atracurium is approximately one third to one half the duration of block by d-tubocurarine, metocurine, and pancuronium at initially equipotent doses. As with other nondepolarizing neuromuscular blockers, the time to onset of paralysis decreases and the duration of maximum effect increases with increasing doses of Atracurium. Repeated administration of maintenance doses of Atracurium has no cumulative effect on the duration of neuromuscular block if recovery is allowed to begin prior to repeat dosing. Moreover, the time needed to recover from repeat doses does not change with additional doses. Repeat doses can therefore be administered at relatively regular intervals with predictable results.
Clinical Use
Atracurium Besylate is metabolized rapidly and nonenzymatically to yield laudanosineand a smaller quaternary compound ,which do not have neuromuscular blocking activity. In vitroexperiments show that atracurium besylate breaks down atpH 7.4 and 37°C by a Hoffman elimination reaction.Atracurium besylate undergoes enzymatic decompositionof its ester function to yield an inactive quaternary alcoholand quaternary acid. AChE inhibitors such as neostigmine,edrophonium, and pyridostigmine antagonize paralysis byatracurium besylate.
Veterinary Drugs and Treatments
Atracurium is indicated as an adjunct to general anesthesia to produce muscle relaxation during surgical procedures or mechanical ventilation and also to facilitate endotracheal intubation. Atracurium can be used in patients with significant renal or hepatic disease.
Drug interactions
Potentially hazardous interactions with other drugs
Anaesthetics: effects enhanced by ketamine;
enhanced effect with volatile liquid general
anaesthetics.
Anti-arrhythmics: procainamide enhances muscle
relaxant effect.
Antibacterials: aminoglycosides, clindamycin,
polymyxin and piperacillin enhance effect of
atracurium.
Antiepileptics: muscle relaxant effects antagonised by
carbamazepine; effects reduced by long-term use of
phenytoin but might be increased by acute use.
Atracurium enhances the neuromuscular block
produced by botulinum toxin (risk of toxicity)
Metabolism
Atracurium besilate undergoes spontaneous degradation via Hofmann elimination (a non-enzymatic breakdown process occurring at physiological pH and temperature) to produce laudanosine and other metabolites. There is also ester hydrolysis by non-specific plasma esterases. The metabolites have no neuromuscular blocking activity. Excretion of atracurium is in urine and bile, mostly as metabolites.
Metabolism
Not Available
Properties of Atracurium besylate
| Melting point: | 85-90°C |
| storage temp. | Inert atmosphere,2-8°C |
| solubility | H2O: ~27 mg/mL |
| form | powder |
| color | white |
| CAS DataBase Reference | 64228-81-5(CAS DataBase Reference) |
Safety information for Atracurium besylate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Atracurium besylate
| InChIKey | YXSLJKQTIDHPOT-UHFFFAOYSA-N |
Atracurium besylate manufacturer
Gland Chemicals Pvt Ltd
Ralington Pharma
Gland Pharma Ltd
BDR Pharmaceuticals International Pvt Ltd
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 4-HYDROXY BENZYL ALCOHOL 3-NITRO-2-METHYL ANILINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 4-methoxy-3,5-dinitropyridine 2-(Cyanocyclohexyl)acetic acid 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 64228-81-5 99%View Details
64228-81-5 99%View Details
64228-81-5 -
 Atracurium besylate 99%View Details
Atracurium besylate 99%View Details
64228-81-5 -
 64228-81-5 Atracurium besylate 98%View Details
64228-81-5 Atracurium besylate 98%View Details
64228-81-5 -
 Atracurium besylate 64228-81-5 98%View Details
Atracurium besylate 64228-81-5 98%View Details
64228-81-5 -
 64228-81-5 98%View Details
64228-81-5 98%View Details
64228-81-5 -
 64228-81-5 98%View Details
64228-81-5 98%View Details
64228-81-5 -
 Atracurium besylate CAS 64228-81-5View Details
Atracurium besylate CAS 64228-81-5View Details
64228-81-5 -
 Atracurium besylate CAS 64228-81-5View Details
Atracurium besylate CAS 64228-81-5View Details
64228-81-5