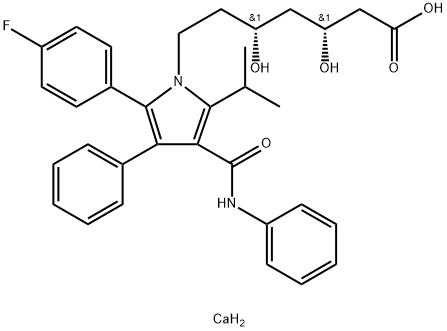
Atorvastatin
- CAS NO.:110862-48-1
- Empirical Formula: C33H35FN2O5
- Molecular Weight: 558.64
- EINECS: 214-486-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-12 15:32:36
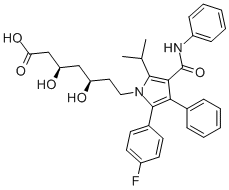
What is Atorvastatin?
Definition
ChEBI: Atorvastatin is a dihydroxy monocarboxylic acid that is a member of the drug class known as statins, used primarily for lowering blood cholesterol and for preventing cardiovascular diseases. It has a role as an environmental contaminant and a xenobiotic. It is an aromatic amide, a member of monofluorobenzenes, a statin (synthetic), a dihydroxy monocarboxylic acid and a member of pyrroles. It is functionally related to a heptanoic acid. It is a conjugate acid of an atorvastatin(1-).
Clinical Use
Hyperlipidaemia and hypercholesterolaemia
Drug interactions
Potentially hazardous interactions with other drugs
Anti-arrhythmics: concentration possibly increased
by dronedarone.
Antibacterials: azithromycin, erythromycin,
clarithromycin or fusidic acid possibly increased
risk of myopathy - avoid atorvastatin for at least
7 days after fusidic acid stopped; concentration
increased by clarithromycin - do not exceed 20 mg
of atorvastatin1
; avoid with telithromycin; increased
risk of myopathy with daptomycin; concentration
possibly reduced by rifampicin.
Anticoagulants: may transiently reduce anticoagulant
effect of warfarin.
Antifungals: increased risk of myopathy with
itraconazole - do not exceed 40 mg of atorvastatin1
;
increased risk of myopathy with fluconazole,
ketoconazole, posaconazole, voriconazole and
possibly other imidazoles and triazoles - avoid.
Antivirals: increased risk of myopathy with
atazanavir, boceprevir (reduce atorvastatin dose),
and possibly darunavir, fosamprenavir, indinavir,
lopinavir, ritonavir, saquinavir or tipranavir (max
dose of atorvastatin 10 mg); concentration reduced
by efavirenz and possibly etravirine; avoid with
dasabuvir, ombitasvir, paritaprevir and telaprevir;
possible increased risk of myopathy with ledipasvir
- reduce atorvastatin dose; concentration increased
by simeprevir - consider reducing atorvastatin
dose.
Calcium channel blockers: concentration increased
by diltiazem - increased risk of myopathy;
concentration of verapamil increased also possible
increased risk of myopathy - consider reducing
atorvastatin dose.
Ciclosporin: increased risk of myopathy - do not
exceed 10 mg of atorvastatin.1 Cobicistat: reduce atorvastatin dose.
Colchicine: possible increased risk of myopathy.
Grapefruit juice: concentration possibly increased.
Lipid lowering agents: increased risk of myopathy
with fibrates, gemfibrozil (avoid) and nicotinic acid.
Metabolism
Atorvastatin undergoes extensive presystemic clearance in gastrointestinal mucosa and/or hepatic first-pass metabolism. Atorvastatin is metabolised by cytochrome P450 3A4 to ortho- and parahydroxylated derivatives and various beta-oxidation products. These products are further metabolised via glucuronidation. Approximately 70% of circulating inhibitory activity for HMG-CoA reductase is attributed to active metabolites. Atorvastatin is eliminated primarily in bile as active metabolites following hepatic and/or extrahepatic metabolism, but does not appear to undergo significant enterohepatic recirculation.
Properties of Atorvastatin
| Boiling point: | 722.2±60.0 °C(Predicted) |
| Density | 1.23±0.1 g/cm3(Predicted) |
| pka | 4.29±0.10(Predicted) |
Safety information for Atorvastatin
Computed Descriptors for Atorvastatin
New Products
1-Boc-4-cyanopiperidine tert-Butyl carbazate 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE TETRABUTYLAMMONIUM CYANIDE TETRAHYDRO-2H-PYRAN-3-OL 3-Pyridineacrylic acid Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% Tadalafil Clopidogrel bisulfate Sitagliptin Phosphate Monohydrate Cabergoline Fexofinadine HCl Etoricoxib 4-Amino Acetophenone 2-Chloro Acetophenone Amlodipine Base 2,3,5-Triiodobenzoic Acid Pyrrolidine Diiodo PentoxideRelated products of tetrahydrofuran

You may like
-
 110862-48-1 ATORVASTATIN 10% DC GRANULES 98%View Details
110862-48-1 ATORVASTATIN 10% DC GRANULES 98%View Details
110862-48-1 -
 110862-48-1 98%View Details
110862-48-1 98%View Details
110862-48-1 -
 366789-02-8 Riveroxaban 98%View Details
366789-02-8 Riveroxaban 98%View Details
366789-02-8 -
 Carvedilol 98%View Details
Carvedilol 98%View Details
72956-09-3 -
 Abiretorone 154229-18-2 98%View Details
Abiretorone 154229-18-2 98%View Details
154229-18-2 -
 73590-58-6 Omeprazole 98%View Details
73590-58-6 Omeprazole 98%View Details
73590-58-6 -
 201530-41-8 Deferasirox 98%View Details
201530-41-8 Deferasirox 98%View Details
201530-41-8 -
 Sertraline HCl 98%View Details
Sertraline HCl 98%View Details
79559-97-0
