Atorvastatin
- CAS NO.:134523-00-5
- Empirical Formula: C33H35FN2O5
- Molecular Weight: 558.65
- MDL number: MFCD00899261
- EINECS: 806-698-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 16:58:18
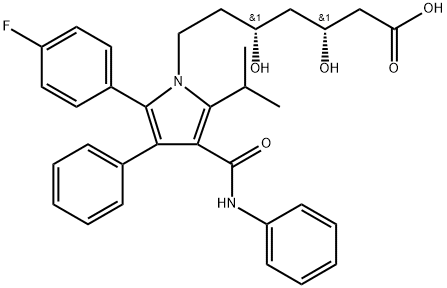
What is Atorvastatin?
Absorption
Atorvastatin presents a dose-dependent and non-linear pharmacokinetic profile. It is very rapidly absorbed after oral administration. After the administration of a dose of 40 mg, its peak plasma concentration of 28 ng/ml is reached 1-2 hours after initial administration with an AUC of about 200 ng?h/ml. Atorvastatin undergoes extensive first-pass metabolism in the wall of the gut and the liver, resulting in an absolute oral bioavailability of 14%. Plasma atorvastatin concentrations are lower (approximately 30% for Cmax and AUC) following evening drug administration compared with morning. However, LDL-C reduction is the same regardless of the time of day of drug administration.
Administration of atorvastatin with food results in prolonged Tmax and a reduction in Cmax and AUC.
Breast Cancer Resistance Protein (BCRP) is a membrane-bound protein that plays an important role in the absorption of atorvastatin. Evidence from pharmacogenetic studies of c.421C>A single nucleotide polymorphisms (SNPs) in the gene for BCRP has demonstrated that individuals with the 421AA genotype have reduced functional activity and 1.72-fold higher AUC for atorvastatin compared to study individuals with the control 421CC genotype. This has important implications for the variation in response to the drug in terms of efficacy and toxicity, particularly as the BCRP c.421C>A polymorphism occurs more frequently in Asian populations than in Caucasians. Other statin drugs impacted by this polymorphism include fluvastatin, simvastatin, and rosuvastatin.
Genetic differences in the OATP1B1 (organic-anion-transporting polypeptide 1B1) hepatic transporter encoded by the SCLCO1B1 gene (Solute Carrier Organic Anion Transporter family member 1B1) have been shown to impact atorvastatin pharmacokinetics. Evidence from pharmacogenetic studies of the c.521T>C single nucleotide polymorphism (SNP) in the gene encoding OATP1B1 (SLCO1B1) demonstrated that atorvastatin AUC was increased 2.45-fold for individuals homozygous for 521CC compared to homozygous 521TT individuals. Other statin drugs impacted by this polymorphism include simvastatin, pitavastatin, rosuvastatin, and pravastatin.
Toxicity
The reported LD50 of oral atorvastatin in mice is higher than 5000 mg/kg. In cases of overdose with atorvastatin, there is reported symptoms of complicated breathing, jaundice, liver damage, dark urine, muscle pain, and seizures. In case of overdose, symptomatic treatment is recommended and due to the high plasma protein binding, hemodialysis is not expected to generate significant improvement.
In carcinogenic studies with high doses of atorvastatin, evidence of rhabdomyosarcoma, fibrosarcoma, liver adenoma, and liver carcinoma were observed.
In fertility studies with high doses of atorvastatin, there were events of aplasia, aspermia, low testis and epididymal weight, decreased sperm motility, decreased spermatid head concentration and increased abnormal sperm.
Atorvastatin was shown to not be mutagenic in diverse mutagenic assays.
Description
Atorvastatin is typically used for lowering cholesterol and has been shown to be effective in preventing cardiovascular disease. Atorvastatin has also shown promise in treating Alzheimer''s disease and preventing melanoma and colon cancer.
Chemical properties
white Crystalline powder
Originator
Cardyl,Pfizer,Spain
The Uses of Atorvastatin
antihyperlipoproteimic;HMG-CoA reductase inhibition
Background
Atorvastatin (Lipitor?), is a lipid-lowering drug included in the statin class of medications. By inhibiting the endogenous production of cholesterol in the liver, statins lower abnormal cholesterol and lipid levels, and ultimately reduce the risk of cardiovascular disease. More specifically, statin medications competitively inhibit the enzyme hydroxymethylglutaryl-coenzyme A (HMG-CoA) Reductase, which catalyzes the conversion of HMG-CoA to mevalonic acid. This conversion is a critical metabolic reaction involved in the production of several compounds involved in lipid metabolism and transport, including cholesterol, low-density lipoprotein (LDL) (sometimes referred to as "bad cholesterol"), and very-low-density lipoprotein (VLDL). Prescribing statins is considered standard practice for patients following any cardiovascular event, and for people who are at moderate to high risk of developing cardiovascular disease. The evidence supporting statin use, coupled with minimal side effects and long term benefits, has resulted in wide use of this medication in North America.
Atorvastatin and other statins including lovastatin, pravastatin, rosuvastatin, fluvastatin, and simvastatin are considered first-line treatment options for dyslipidemia. The increasing use of this class of drugs is largely attributed to the rise in cardiovascular diseases (CVD) (such as heart attack, atherosclerosis, angina, peripheral artery disease, and stroke) in many countries. An elevated cholesterol level (elevated low-density lipoprotein (LDL) levels in particular) is a significant risk factor for the development of CVD. Several landmark studies demonstrate that the use of statins is associated with both a reduction in LDL levels and CVD risk. Statins were shown to reduce the incidences of all-cause mortality, including fatal and non-fatal CVD, as well as the need for surgical revascularization or angioplasty following a heart attack. Some evidence has shown that even for low-risk individuals (with <10% risk of a major vascular event occurring within five years) statin use leads to a 20%-22% relative reduction in the number of major cardiovascular events (heart attack, stroke, coronary revascularization, and coronary death) for every 1 mmol/L reduction in LDL without any significant side effects or risks.
Atorvastatin was first synthesized in 1985 by Dr. Bruce Roth and approved by the FDA in 1996. It is a pentasubstituted pyrrole formed by two contrasting moieties with an achiral heterocyclic core unit and a 3,5-dihydroxypentanoyl side chain identical to its parent compound. Unlike other members of the statin group, atorvastatin is an active compound and therefore does not require activation.
What are the applications of Application
Atorvastatin is a potent, selective and competitive HMG-CoA reductase inhibitor, with oral bioavailability.
Indications
Atorvastatin is indicated for the treatment of several types of dyslipidemias, including primary hyperlipidemia and mixed dyslipidemia in adults, hypertriglyceridemia, primary dysbetalipoproteinemia, homozygous familial hypercholesterolemia, and heterozygous familial hypercholesterolemia in adolescent patients with failed dietary modifications.
Dyslipidemia describes an elevation of plasma cholesterol, triglycerides or both as well as to the presence of low levels of high-density lipoprotein. This condition represents an increased risk for the development of atherosclerosis.
Atorvastatin is indicated, in combination with dietary modifications, to prevent cardiovascular events in patients with cardiac risk factors and/or abnormal lipid profiles.
Atorvastatin can be used as a preventive agent for myocardial infarction, stroke, revascularization, and angina, in patients without coronary heart disease but with multiple risk factors and in patients with type 2 diabetes without coronary heart disease but multiple risk factors.
Atorvastatin may be used as a preventive agent for non-fatal myocardial infarction, fatal and non-fatal stroke, revascularization procedures, hospitalization for congestive heart failure and angina in patients with coronary heart disease.
Prescribing of statin medications is considered standard practice following any cardiovascular events and for people with a moderate to high risk of development of CVD. Statin-indicated conditions include diabetes mellitus, clinical atherosclerosis (including myocardial infarction, acute coronary syndromes, stable angina, documented coronary artery disease, stroke, trans ischemic attack (TIA), documented carotid disease, peripheral artery disease, and claudication), abdominal aortic aneurysm, chronic kidney disease, and severely elevated LDL-C levels.
Definition
ChEBI: Atorvastatin is a dihydroxy monocarboxylic acid that is a member of the drug class known as statins, used primarily for lowering blood cholesterol and for preventing cardiovascular diseases. It has a role as an environmental contaminant and a xenobiotic. It is an aromatic amide, a member of monofluorobenzenes, a statin (synthetic), a dihydroxy monocarboxylic acid and a member of pyrroles. It is functionally related to a heptanoic acid. It is a conjugate acid of an atorvastatin(1-).
Manufacturing Process
285 ml 2.2 M n-butyl lithium in hexane was added dropwise to 92 ml
diisopropylamine at -50-60°C under nitrogen. The well stirred solutions
warmed to about -20°C, then it was cannulated into a suspension of 99 g of
S(+)-2-acetoxy-1,1,2-triphenylethanol in 500 ml absolute tetrahydrophuran
(THF) at -70°C and the reaction mixture was allowed to warm to -10°C for 2
hours. A suspension of MgBr2 was made from 564 ml (0.63 mol) of bromine
and 15.3 g of magnesium (0.63 mol) in 500 ml THF cooled to -78°C. The
enolate solution was cannulated into this suspension within 30 min and was
stirred for 60 min at -78°C. 150 g 5-(4-fluorophenyl)-2-(1-methylethyl)-1-(3-
oxopropyl)-N,4-diphenyl-1H-pyrrole-3-carboxamide in 800 ml absolute THF
was added dropwise over 30 min, stirred 90 min at -78°C, then was added
200 ml acetic acid, this is removed to a cool bath, 500 ml of H2O was added
and the mixture concentrate in vacuo at 40-50°C. After adding of 500 ml of
1:1 EtOAc/heptane the mixture was filtered. The filtrate was washed
extensively with 0.5 N HCl, then several times with H2O and finally EtOAc/heptane (3:1) and cooled with dry ice to -20°C. The light brown
crystalline product was dried in vacuum oven at 40°C. The yield was 194 g.
112 g of the same product was produced by evaporation of mother liquor
after recrystallization and chromatographic purification on a silicagel.
162 g of this substance was suspended in methanol/THF (5:3) and was stirred
with 11.7 g of sodium methoxide until everything was dissolved and kept in
the freezer overnight. Later it was quenched with AcOH concentrated in vacuo,
was added to 500 ml H2O and extracted twice with EtOAc (300 ml). The
combined extracts was washed with saturated NaHCO3 brine and dried over
anhydrous MgSO4, purified on silica-gel and gave 86.1 g of white crystals m.p.
125-126°C, αD
20=4.23° (1.17 M, CH3OH).
81 g of the last product in 500 ml absolute THF was added as quickly as
possible to the mixture of 77 ml THF at diisopropylamine, 200 ml 2.2 M of nbutyl
lithium and 62 ml of t-butylacetate in 200 ml THF -40-42°C under
nitrogen. Stirring was continued for 4 hours at -70°C. The reaction mixture
was concentrated in vacuo, the residue was taken up in EtOAc, washed with
water, then saturated NH4Cl, NaHCO3 (saturated), dried over anhydrous
MgSO4, filtred and the solvent evaporated.
The organic phase was dried and concentrated in vacuo to yield 73 g crude
product, that was dissolved in 500 ml absolute THF, 120 ml triehtylborane and
0.7 g t-butylcarboxylic acid, 70 ml methanol and 4.5 g sodium borohydride
was added. The mixture was stirred at -78°C under a dry atmosphere for 6
hours, poured slowly into 4:1:1 mixture of ice/30%H2O2/H2O and stirred
overnight. CHCl3 (400 ml) was added and organic layer washed extensively
with H2O until no peroxide could be found, was dried over MgSO4, filtered and
was treated by chromatography on silica gel to yield 51 g. The product was
dissolved in THF/methanol and saponificated with NaOH and, concentrated to
remove organic solvents at room temperature, added 100 ml H2O, and
extracted with Et2O twice. Organic layer was thoroughly dried and it was left
at room temperature for the next 10 days, then concentrated.
Chromatography on silica gel yielded 13.2 g racemate of lactone - trans-(+/-
)-5-(4-fluorophenyl)-2-(1-methylethyl)-N,4-di-diphenyl-1-[2-(tetrahydro-4-
hydroxy-6-oxo-2H-pyran-2-yl)ethyl]-1H-pyrrole-3-carboxamide. This racemate
was divided by chiral synthesis which was made analogously the method in US
Pat. No. 4,581,893. Then each isomer was saponificated with NaOH and
purificated by HPLC. The calcium salt corresponding acid was prepared by
reaction with 1 eq. of CaCl2·2H2O in water.
brand name
Lipitor (Pfizer).
Therapeutic Function
Anticholesteremic
General Description
Atorvastatin, [R-(R*,R*)]-2-(4-fluorophenyl)-b,d-dihydroxy-5-(1-methylethyl)-3-phenyl-4-[(phenylamino)carbonyl]-lH-pyrrole-1-heptanoic acid(Lipitor), also possesses the heptanoic acid side chain,which is critical for inhibition of HMG-CoA reductase.Although the side chain is less lipophilic than the lactoneform, the high amount of lipophilic substitution causes this agent to have a slightly higher level of CNS penetration thanpravastatin, resulting in a slight increase in CNS side effects.Even so, its CNS profile is much lower than that of lovastatin.Atorvastatin is marketed as a combination therapywith amlodipine under the trade name Norvasc for managementof high cholesterol and high blood pressure.
General Description
Cerivastatin (Baycol) is one of the neweragents in this class of cholesterol-lowering agents. However,it carries a higher incidence of rhabdomyolysis and, as a result,was voluntarily withdrawn from the market by its manufacturerin 2001.
Pharmacokinetics
Atorvastatin is an oral antilipemic agent that reversibly inhibits HMG-CoA reductase. It lowers total cholesterol, low-density lipoprotein-cholesterol (LDL-C), apolipoprotein B (apo B), non-high density lipoprotein-cholesterol (non-HDL-C), and triglyceride (TG) plasma concentrations while increasing HDL-C concentrations. High LDL-C, low HDL-C and high TG concentrations in the plasma are associated with increased risk of atherosclerosis and cardiovascular disease. The total cholesterol to HDL-C ratio is a strong predictor of coronary artery disease, and high ratios are associated with a higher risk of disease. Increased levels of HDL-C are associated with lower cardiovascular risk. By decreasing LDL-C and TG and increasing HDL-C, atorvastatin reduces the risk of cardiovascular morbidity and mortality.
Elevated cholesterol levels (and high low-density lipoprotein (LDL) levels in particular) are an important risk factor for the development of CVD. Clinical studies have shown that atorvastatin reduces LDL-C and total cholesterol by 36-53%. In patients with dysbetalipoproteinemia, atorvastatin reduced the levels of intermediate-density lipoprotein cholesterol. It has also been suggested that atorvastatin can limit the extent of angiogenesis, which can be useful in the treatment of chronic subdural hematoma.
Myopathy/Rhabdomyolysis
Atorvastatin, like other HMG-CoA reductase inhibitors, is associated with a risk of drug-induced myopathy characterized by muscle pain, tenderness, or weakness in conjunction with elevated levels of creatine kinase (CK). Myopathy often manifests as rhabdomyolysis with or without acute renal failure secondary to myoglobinuria. The risk of statin-induced myopathy is dose-related, and the symptoms of myopathy are typically resolved upon drug discontinuation. Results from observational studies suggest that 10-15% of people taking statins may experience muscle aches at some point during treatment.
Liver Dysfunction
Statins, like some other lipid-lowering therapies, have been associated with biochemical abnormalities of liver function. Persistent elevations (> 3 times the upper limit of normal [ULN] occurring on two or more occasions) in serum transaminases occurred in 0.7% of patients who received atorvastatin in clinical trials. This effect appears to be dose-related.
Endocrine Effects
Statins are associated with a risk of increased serum HbA1c and glucose levels. An in vitro study demonstrated a dose-dependent cytotoxic effect on human pancreatic islet β cells following treatment with atorvastatin. Moreover, insulin secretion rates decreased relative to control.
HMG-CoA reductase inhibitors interfere with cholesterol synthesis and may theoretically interfere with the production of adrenal and/or gonadal steroids. Clinical studies with atorvastatin and other HMG-CoA reductase inhibitors have suggested that these agents do not affect plasma cortisol concentrations, basal plasma testosterone concentration, or adrenal reserve. However, the effect of statins on male fertility has not been fully investigated. The effects of statins on the pituitary-gonadal axis in premenopausal women are unknown.
Cardiovascular
Significant decreases in circulating ubiquinone levels in patients treated with atorvastatin and other statins have been observed. The clinical significance of a potential long-term statin-induced deficiency of ubiquinone has not been established. It has been reported that a decrease in myocardial ubiquinone levels could lead to impaired cardiac function in patients with borderline congestive heart failure.
Lipoprotein A
In some patients, the beneficial effect of lowered total cholesterol and LDL-C levels may be partly blunted by the concomitant increase in Lp(a) lipoprotein concentrations. Present knowledge suggests the importance of high Lp(a) levels as an emerging risk factor for coronary heart disease. Further studies have demonstrated statins affect Lp(a) levels differently in patients with dyslipidemia depending on their apo(a) phenotype; statins increase Lp(a) levels exclusively in patients with the low molecular weight apo(a) phenotype.
Metabolism
Atorvastatin is highly metabolized to ortho- and parahydroxylated derivatives and various beta-oxidation products, primarily by Cytochrome P450 3A4 in the intestine and liver. Atorvastatin's metabolites undergo further lactonization via the formation of acyl glucuronide intermediates by the enzymes UGT1A1 and UGT1A3. These lactones can be hydrolyzed back to their corresponding acid forms and exist in equilibirum.
In vitro inhibition of HMG-CoA reductase by ortho- and parahydroxylated metabolites is equivalent to that of atorvastatin. Approximately 70% of circulating inhibitory activity for HMG-CoA reductase is attributed to active metabolites.
Properties of Atorvastatin
| Melting point: | 176-178°C |
| Boiling point: | 722.2±60.0 °C(Predicted) |
| Density | 1.23±0.1 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | DMSO: 100 mg/mL (179.01 mM) |
| pka | pKa 4.46(H2O t=30 Iuncontrolled) (Uncertain) |
| form | Solid |
| color | White to light yellow |
| CAS DataBase Reference | 134523-00-5(CAS DataBase Reference) |
| EPA Substance Registry System | 1H-Pyrrole-1-heptanoic acid, 2-(4-fluorophenyl)-?,?-dihydroxy-5-(1-methylethyl)-3-phenyl-4-[(phenylamino)carbonyl]-, (?R,?R)- (134523-00-5) |
Safety information for Atorvastatin
| Signal word | Warning |
| Pictogram(s) |
 Flame Over Circle Oxidizers GHS03  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H272:Oxidising liquids;Oxidising solids H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P221:Take any precaution to avoid mixing with combustibles/… P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P312:Call a POISON CENTER or doctor/physician if you feel unwell. P380:Evacuate area. P302+P352:IF ON SKIN: wash with plenty of soap and water. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P370+P380+P375:in case of fire: Evacuate area. Fight fire remotely due to the risk of explosion. |
Computed Descriptors for Atorvastatin
Atorvastatin manufacturer
Clickchem Research LLP
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran




You may like
-
 134523-00-5 Atorvastatin 98%View Details
134523-00-5 Atorvastatin 98%View Details
134523-00-5 -
 Atorvastatin 98%View Details
Atorvastatin 98%View Details -
 Thiosalicylic acid 99%View Details
Thiosalicylic acid 99%View Details
134523-00-5 -
 Atorvastatin 99%View Details
Atorvastatin 99%View Details -
 Atorvastatin 99%View Details
Atorvastatin 99%View Details -
 134523-00-5 98%View Details
134523-00-5 98%View Details
134523-00-5 -
 134523-00-5 Atorvastatin 98%View Details
134523-00-5 Atorvastatin 98%View Details
134523-00-5 -
 134523-00-5 99%View Details
134523-00-5 99%View Details
134523-00-5
