Atorvastatin calcium
Synonym(s):(3 R,5 R)-7-(2-(4-Fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)-1H-pyrrol-1-yl)-3,5-dihydroxyheptanoate, hemicalcium salt;(3R,5R)-7-(2-(4-Fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)-1H-pyrrol-1-yl)-3,5-dihydroxyheptanoate, hemicalcium salt;(betaR,deltaR)-2-(p-Fluorophenyl)-beta,delta-dihydroxy-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrole-1-heptanoate, calcium salt (2:1) trihyddrate;[R-(R*, R*)]-2-(4-Fluorophenyl)-β, δ-dihydroxy-5-(1-methylethyl)-3-phenyl-4-[(phenylamino)carbonyl]-1Hpyrrole-1-heptanoic acid, calcium salt (2:1) trihydrate;1H-Pyrrole-1-heptanoic acid, [R-(R*,R*)]-2-(4-flurophenyl)-β,δ-dihydroxy-5-(1-methylethyl)-3-phenyl-4-[(phenylamino)carbonyl], calcium salt (2:1)
- CAS NO.:134523-03-8
- Empirical Formula: C33H37CaFN2O5
- Molecular Weight: 600.74
- MDL number: MFCD03613598
- EINECS: 200-659-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 16:58:18
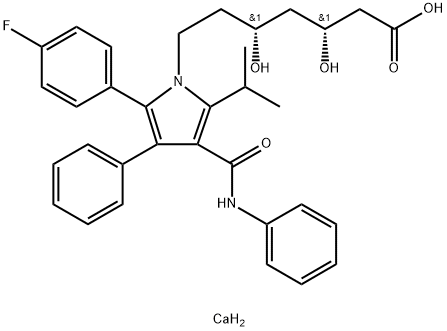
What is Atorvastatin calcium?
Description
Lipitor was launched in Canada, the Netherlands, the UK and the US as an orally-active hypocholesterolemic agent. It was the first pharmaceutical product ever to attain over one billion dollars in sales in its first year. It can be synthesized by a number of routes but the most efficient involves the Paal-Knorr reaction of an acetonide protected dihydroxy amino ester and a diaryl phenylacetamide diketone. Lipitor is a liver selective, reversible competitive inhibitor of HMG-CoA reductase, the rate limiting step in cholesterol biosynthesis. Lipitor monotherapy resulted in a reduction of LDL cholesterol by up to 60%. Lipitor is about 2-4 times more potent, on a dosage basis, than Simvastatin. The superior properties of Lipitor can be attributed to its greater uptake and longer duration of action in the liver. In addition to its effects on cholesterol, Lipitor is also effective in lowering triglycerides. While the mechanism is not clear, two theories proposed are: a) the decrease in cholesterol causes a concomitant increase in hepatic LDL-receptor activity (mostly B and E type) which results in a decrease in triglycerides through an increase in binding of triglycerides to VLDL and LDL, and b) the decreased level of cholesterol impairs VLDL transport of triglycerides.
Chemical properties
White Crystalline Powder
Physical properties
Atorvastatin calcium is a white to off-white crystalline powder that is insoluble in aqueous solutions of pH 4and below. Atorvastatin calcium is very slightly soluble in distilled water, pH 7.4 phosphate buffer, and acetonitrile; slightly soluble in ethanol; and freely soluble in methanol.
Originator
Parke-Davis (US)
The Uses of Atorvastatin calcium
Atorvastatin Calcium Salt Trihydrate is a compound used as standard in the method development and validation for simultaneous determination of Atorvastatin calcium (A791750) and Ezetimibe (E975000) in tablets using UV spectrophotometric, HPLC and HPTLC methods.
The Uses of Atorvastatin calcium
A selective, competitive HMG-CoA reductase inhibitor. The only drug in its class specfically indicated for lowering both elevated LDL-cholesterol and triglycerides in patients with hypercholesterolemia
The Uses of Atorvastatin calcium
A selective, competitive HMG-CoA reductase inhibitor. Atorvastatin is the only drug in its class specfically indicated for lowering both elevated LDL-cholesterol and triglycerides in patients with hypercholesterolemia.
The Uses of Atorvastatin calcium
antihyperlipidemic, HMGCoA reductase inhibitor
The Uses of Atorvastatin calcium
DMSO and EtOH soluble
The Uses of Atorvastatin calcium
The primary uses of atorvastatin is for the treatment of dyslipidemia and the prevention of cardiovascular disease.
Definition
ChEBI: An organic calcium salt composed of calcium cations and atorvastatin anions in a 1:2 ratio.
Manufacturing Process
285 ml 2.2 M n-butyl lithium in hexane was added dropwise to 92 ml
diisopropylamine at -50-60°C under nitrogen. The well stirred solutions
warmed to about -20°C, then it was cannulated into a suspension of 99 g of
S(+)-2-acetoxy-1,1,2-triphenylethanol in 500 ml absolute tetrahydrophuran
(THF) at -70°C and the reaction mixture was allowed to warm to -10°C for 2
hours. A suspension of MgBr2 was made from 564 ml (0.63 mol) of bromine
and 15.3 g of magnesium (0.63 mol) in 500 ml THF cooled to -78°C. The
enolate solution was cannulated into this suspension within 30 min and was
stirred for 60 min at -78°C. 150 g 5-(4-fluorophenyl)-2-(1-methylethyl)-1-(3-
oxopropyl)-N,4-diphenyl-1H-pyrrole-3-carboxamide in 800 ml absolute THF
was added dropwise over 30 min, stirred 90 min at -78°C, then was added
200 ml acetic acid, this is removed to a cool bath, 500 ml of H2O was added
and the mixture concentrate in vacuo at 40-50°C. After adding of 500 ml of
1:1 EtOAc/heptane the mixture was filtered. The filtrate was washed
extensively with 0.5 N HCl, then several times with H2O and finally EtOAc/heptane (3:1) and cooled with dry ice to -20°C. The light brown
crystalline product was dried in vacuum oven at 40°C. The yield was 194 g.
112 g of the same product was produced by evaporation of mother liquor
after recrystallization and chromatographic purification on a silicagel.
162 g of this substance was suspended in methanol/THF (5:3) and was stirred
with 11.7 g of sodium methoxide until everything was dissolved and kept in
the freezer overnight. Later it was quenched with AcOH concentrated in vacuo,
was added to 500 ml H2O and extracted twice with EtOAc (300 ml). The
combined extracts was washed with saturated NaHCO3 brine and dried over
anhydrous MgSO4, purified on silica-gel and gave 86.1 g of white crystals m.p.
125-126°C, αD
20=4.23° (1.17 M, CH3OH).
81 g of the last product in 500 ml absolute THF was added as quickly as
possible to the mixture of 77 ml THF at diisopropylamine, 200 ml 2.2 M of nbutyl
lithium and 62 ml of t-butylacetate in 200 ml THF -40-42°C under
nitrogen. Stirring was continued for 4 hours at -70°C. The reaction mixture
was concentrated in vacuo, the residue was taken up in EtOAc, washed with
water, then saturated NH4Cl, NaHCO3 (saturated), dried over anhydrous
MgSO4, filtred and the solvent evaporated.
The organic phase was dried and concentrated in vacuo to yield 73 g crude
product, that was dissolved in 500 ml absolute THF, 120 ml triehtylborane and
0.7 g t-butylcarboxylic acid, 70 ml methanol and 4.5 g sodium borohydride
was added. The mixture was stirred at -78°C under a dry atmosphere for 6
hours, poured slowly into 4:1:1 mixture of ice/30%H2O2/H2O and stirred
overnight. CHCl3 (400 ml) was added and organic layer washed extensively
with H2O until no peroxide could be found, was dried over MgSO4, filtered and
was treated by chromatography on silica gel to yield 51 g. The product was
dissolved in THF/methanol and saponificated with NaOH and, concentrated to
remove organic solvents at room temperature, added 100 ml H2O, and
extracted with Et2O twice. Organic layer was thoroughly dried and it was left
at room temperature for the next 10 days, then concentrated.
Chromatography on silica gel yielded 13.2 g racemate of lactone - trans-(+/-
)-5-(4-fluorophenyl)-2-(1-methylethyl)-N,4-di-diphenyl-1-[2-(tetrahydro-4-
hydroxy-6-oxo-2H-pyran-2-yl)ethyl]-1H-pyrrole-3-carboxamide. This racemate
was divided by chiral synthesis which was made analogously the method in US
Pat. No. 4,581,893. Then each isomer was saponificated with NaOH and
purificated by HPLC. The calcium salt corresponding acid was prepared by
reaction with 1 eq. of CaCl2·2H2O in water.
Side Effects
Get emergency medical help if you have signs of an allergic reaction to this medicine (hives, difficult breathing, swelling in your face or throat) or a severe skin reaction (fever, sore throat, burning eyes, skin pain, red or purple skin rash with blistering and peeling).
Atorvastatin calcium can cause the breakdown of muscle tissue, which can lead to kidney failure.
Muscle problems may be more likely in older adults and those who have kidney problems, thyroid problems, or take certain other medicines.
brand name
Lipitor (Pfizer).
Therapeutic Function
Anticholesteremic
Biological Activity
Potent HMG-CoA reductase inhibitor (IC 50 = 8 nM). Reduces circulating LDL-C by inhibiting cholesterol biosynthesis and inducing expression of LDL receptors. Inhibits smooth muscle cell proliferation in vitro and exhibits antinociceptive effects in the inflammatory hypernociception model.
Properties of Atorvastatin calcium
| Melting point: | 176-178°C |
| storage temp. | Keep in dark place,Inert atmosphere,2-8°C |
| solubility | DMSO: ≥10mg/mL |
| form | white powder |
| color | White to Off-White |
| CAS DataBase Reference | 134523-03-8(CAS DataBase Reference) |
| EPA Substance Registry System | 1H-Pyrrole-1-heptanoic acid, 2-(4-fluorophenyl)-.beta.,delta.-dihydroxy-5-(1-methylethyl)-3-phenyl-4-[(phenylamino)carbonyl]-, calcium salt (2:1),(.beta.R,.delta.R)- (134523-03-8) |
Safety information for Atorvastatin calcium
Computed Descriptors for Atorvastatin calcium
| InChIKey | FQCKMBLVYCEXJB-MNSAWQCASA-L |
Atorvastatin calcium manufacturer
SRINI PHARMACEUTICALS PVT LTD
Indexim International
Eastern Chemicals Mumbai Private Limited
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
![3S,5R isoMer, or (3S,5R)-7-[3-(phenylcarbaMoyl)-5-(4-fluorophenyl)-2-isopropyl-4-phenyl-1H-pyrrol-1-yl]-3,5-dihydroxyheptanoic acid calciuM salt](https://img.chemicalbook.in/CAS/GIF/887196-25-0.gif)

You may like
-
 Atorvastatin Calcium 99%View Details
Atorvastatin Calcium 99%View Details -
 Atorvastatin Calcium 98%View Details
Atorvastatin Calcium 98%View Details -
 Atorvastatin Calcium 98%View Details
Atorvastatin Calcium 98%View Details -
 Atorvastatin Calcium CAS-134523-03-8, Packaging Size:25kg, Grade Standard: pharmaView Details
Atorvastatin Calcium CAS-134523-03-8, Packaging Size:25kg, Grade Standard: pharmaView Details
134523-03-8 -
 Atorvastatin Calcium ApiView Details
Atorvastatin Calcium ApiView Details
134523-00-5 -
 Atorvastatin Calcium API Powder USPView Details
Atorvastatin Calcium API Powder USPView Details
134523-00-5 -
 Atorvastatin Calcium Api, Grade Standard: Medicine Grade, 134523-00-5View Details
Atorvastatin Calcium Api, Grade Standard: Medicine Grade, 134523-00-5View Details
134523-00-5 -
 Atorvastatin Calcium Api IP/BP/USP/EPView Details
Atorvastatin Calcium Api IP/BP/USP/EPView Details
134523-00-5
