ARSINE
- CAS NO.:7784-42-1
- Empirical Formula: AsH3
- Molecular Weight: 77.94542
- MDL number: MFCD00003432
- EINECS: 232-066-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-06 13:21:43
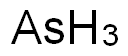
What is ARSINE?
Description
Arsine is a colorless, extremely toxic, flammable
gas at room temperature and atmospheric
pressure and is heavier than air. It has a mild
garliclike odor and acts as a blood and nerve
poison. It can be fatal if inhaled in sufficient
quantity and can form flammable mixtures with
air.
Arsine is shipped as a liquefied compressed
gas in steel cylinders under its own vapor pressure
of 219.7 psia (1515 kPa, abs). Arsine is
slightly soluble in both water and organic solvents.
It reacts readily with agents such as potassium
permanganate, bromine, and sodium
hypochlorite to form arsenic compounds. Arsine
is stable at room temperature, but begins to decompose
into its elements around 446°F to
464°F (230°C to 240°C).
Chemical properties
colourless gas smelling of garlic
Chemical properties
Arsine is a highly toxic, colorless gas with a garlic odor. It is soluble in water, benzene, and chloroform. It is extremely flammable and explosive when exposed to heat, sparks, or flames. Arsine decomposes on heating and under the infl uence of light and moisture, producing toxic arsenic fumes. Arsine reacts with strong oxidants, causing explosion hazard and may explosively decompose on shock, friction, or concussion. Workers in the metallurgical industry involved in the production process and the maintenance of furnaces, and in the microelectronics industry get exposed to the substance. Arsine is extensively used in semiconductor industries, and in the manufacture of microchips.
Chemical properties
Arsine (SA) is an extremely flammable and poisonous, colorless, liquefied compressed gas. Slight garlic-like odor. SA is nonirritating, produces no immediate symptoms, and odor is not an adequate indicator of arsine’s (SA) presence, so persons exposed to hazardous levels may be unaware of its presence. The established PEL is 10 times lower than the concentration at which people begin to smell SA (odor threshold of 0.5 ppm and above).
Physical properties
Colorless gas; garlic-like unpleasant odor; liquefies at -55°C; solidifies at -116.3°C; heavier than air; gas density 2.695 (air =1); sparingly soluble in cold water (~ 20 mg/100 g water or about 640 mg/L at the NTP); soluble in chloroform and benzene.
The Uses of ARSINE
Arsine (AsH3), as a colorless gas, is also known as arsenic hydride. It is used to synthesize organic compounds and as the major ingredient of several military poisons, including the wartime gas lewisite.
The Uses of ARSINE
Organic synthesis, military poison, doping agent for solid-state electronic components.
The Uses of ARSINE
It is used as a doping agent for solidstateelectronic components and as a militarypoison gas. Risk of exposure may arise whenan arsenic compound reacts with an acid ora strong alkali.
Definition
A poisonous colorless gas with an unpleasant smell. It decomposes to arsenic and hydrogen at 230°C. It is produced in the analysis for arsenic (Marsh’s test).
Preparation
Arsine is produced by the reaction of arsenic trichloride, arsenic trioxide or
any inorganic arsenic compound with zinc and sulfuric acid. It is also made by
treating a solution of sodium arsenide or potassium arsenide in liquid ammonia
with ammonium bromide:
Na3As + 3NH4Br → AsH3 + 3NaBr + 3NH3
It may be also prepared by decomposition of alkali metal arsenides by water;
or arsenides of other metals with acids:
Ca3As2 + 6 HCl → 2 AsH3 + 3 CaCl2
A poor yield may be obtained if water is substituted for acids. Thus calcium arsenide reacts with water to produce about 15% arsine.
Air & Water Reactions
Highly flammable. On exposure to light, decomposes rapidly depositing shiny black arsenic.
Reactivity Profile
ARSINE decomposes into its elements (arsenic, gaseous hydrogen) when heated to 300°C. Can form accidentally by the reaction of arsenic impurities with mineral acids (hydrochloric acid, sulfuric acid) in the presence of common metals (iron, zinc). A reducing agent---not oxidized by air at room temperature [Kirk-Othmer, 3rd ed., Vol. 3, 1978, p. 251], but may react vigorously with other oxidizing agents [Sax, 9th ed., 1996, p. 279]. Moderately explosive in combination with chlorine or nitric acid. When heated to decomposition or ignited, ARSINE emits highly toxic fumes of metallic arsenic.
Hazard
Highly poisonous by inhalation. Periph- eral nervous system and vacular system impairment, kidney and liver impairment.
Health Hazard
ARSINE is highly toxic by inhalation; a very short exposure to small quantities may cause death or permanent injury. ARSINE is the most powerful hemolytic poison encountered in industry.
Health Hazard
Arsine is a highly toxic gas. It primarily targets the erythrocyte (red blood cell) and rapidly induces intravascular hemolysis. Secondary effects resulting from hemolysis include hemolytic anemia, hepatic and renal damage. The exact mechanism by which arsenic causes erythrocytes to rupture is unknown, but it is believed to be due to either oxidative damage or a reaction with sulfydryl. As stated, arsine is a potent hemolytic agent and causes acute intravascular hemolysis, rapid red blood cell destruction, and renal failure. Arsine is highly soluble in body fat and hence, can easily cross the alveolo-capillary membrane and into the red blood cell. Arsine causes chemical burns. Exposures to arsine cause headaches, malaise, weakness, dizziness, dyspnea; abdomen and back pain;nausea, vomiting, diarrhea, bronze skin; hematuria (hemoglobin in urine), jaundice, liver enlargement, fever, anxiety, disorientation, delirium, shivering, muscular cramps, tachypnea, tachycardia, anemia, hyperkalemia, electro-cardiographic changes, burning sensations, peripheral neuropathy (focal anesthesia and paresthesia), agitation, disorientation, and hallucinations. The exposed individual and/or the occupational worker soon develops a sensation of cold and paresis in the limbs, hemoglobinuria, a garlic-like odor to the breath, multi-organ failure, and massive hemolysis and kidney failure. Toxic pulmonary edema or acute circulatory failure has been reported as the cause of death in some cases of arsine poisoning. Studies have indicated that occupational exposures to arsine cause increased rates of miscarriage among women associated with the semiconductor industry.
Health Hazard
The acute toxicity of arsine by inhalation is extremely high. This substance is a powerful systemic toxin with a strong affinity for the hemoglobin in the blood, causing hemolysis. Acute inhalation of arsine can cause the breakdown of red blood cells and hemoglobin, impairment of kidney function, damage to the liver and heart, electroencephalogram abnormality, hemolytic anemia, and death due to kidney or heart failure. Symptoms may be delayed for several hours, particularly if very low concentrations have been inhaled. Symptoms of exposure to arsine may include headache, malaise, weakness, dizziness, breathing difficulty, abdominal pain, nausea, vomiting, jaundice, dark red (bloody) urine followed by absence of urination, pulmonary edema, and coma. Exposure to a concentration of 5 to 10 ppm in air for several minutes may be hazardous to human health. The minimum amount of arsine detectable by odor is about 0.5 ppm; since the permissible exposure limit is 0.05 ppm, arsine does not have adequate warning properties to avoid overexposure. In cases where the amount of inhaled arsine is insufficient to produce acute effects, or where small quantities are inhaled over prolonged periods, destruction of red blood cells will occur. The only symptoms noted may be general tiredness, pallor, breathlessness on exertion, and palpitations as would be expected with severe secondary anemia. The carcinogenicity of arsine in humans has not been established; however, arsenic and certain inorganic arsenic compounds are recognized human carcinogens.
Health Hazard
Arsine is a highly poisonous gas showingsevere acute and chronic toxicity in test animalsas well as in humans. Exposure to aconcentration of 5–10 ppm in air for severalminutes may be hazardous to human health.There are conflicting reports on the concentrationsat which arsine is fatal to humans.However, an exposure to 100 ppm in air for1 hour should be lethal. The LC50 valuesdetermined on animals varied according tothe species. The symptoms of acute toxicityare headache, weakness, dizziness, vomiting,abdominal pain, and dyspnea. Arsenicis excreted in urine following exposure.In severe cases of acute poisoning, deathcan result from renal failure and pulmonaryedema. Chronic poisoning can lead to jaundice,hemoglobinuria, hemolysis, and renaldamage. The target organs are the blood, kidneys,and liver. Arsine is a cancer-causinggas. There is sufficient evidence of its carcinogenicityin humans.
Fire Hazard
Vapors may travel to a source of ignition and flash back. Container may explode in heat of fire. When heated to decomposition, emits highly toxic fumes. Can react vigorously with oxidizing materials. May explode when exposed to chlorine, nitric acid, or potassium plus ammonia. On exposure to light, moist ARSINE decomposes quickly, depositing shiny black arsenic.
Fire Hazard
Arsine is flammable in air, having a lower explosion limit (LEL) of 5.8%. The upper limit has not been determined. Combustion products (arsenic trioxide and water) are less toxic than arsine itself. In the event of an arsine fire, stop the flow of gas if possible without risk of harmful exposure and let the fire burn itself out.
Flammability and Explosibility
Arsine is flammable in air, having a lower explosion limit (LEL) of 5.8%. The upper limit has not been determined. Combustion products (arsenic trioxide and water) are less toxic than arsine itself. In the event of an arsine fire, stop the flow of gas if possible without risk of harmful exposure and let the fire burn itself out.
Materials Uses
Arsine is noncorrosive and may, therefore, be used with most of the commercially available metals. However, since arsine is mainly used for the electronics industry, stainless steel is recommended for the gas delivery systems. Stainless steel regulators should be used for all highpurity applications with arsine and arsine mixtures.
Safety Profile
Confirmed human carcinogen. Poison by inhalation. Human red blood cell, gastrointestinal system, central nervous system, and other systemic effects by inhalation. Flammable when exposed to flame. Moderately explosive when exposed to Cl2, HNO3, (K + NH3, open flame, or powerful shock. Dangerous, more toxic than its oxidation product. When heated to decomposition it emits highly toxic fumes of arsenic. See also ARSENIC, ARSENIC COMPOUNDS, and HYDRIDES.
Potential Exposure
Arsine is used in making electronic, semiconductor components; in organic syntheses; and in making lead-acid storage batteries. Arsine may be generated by side reactions or unexpectedly; e.g., it may be generated in metal pickling operations; metal drossing operations; or when inorganic arsenic compounds contact sources of nascent hydrogen. It has been known to occur as an impurity in acetylene. Most occupational exposure occurs in chemical, smelting, and refining industries. It has been used as a poison gas. Cases of exposure have come from workers dealing with zinc, tin, cadmium, galvanized coated aluminum; and silicon and steel metals. A regulated, marked area should be established where this chemical is handled, used, or stored in compliance with OSHA Standard 1910.1045. SA is used as a military poison gas (blood agent). It forms cyanide in the body.
Physiological effects
Arsine is an extremely toxic gas that attacks the
nervous and circulatory systems and can be fatal
if inhaled in sufficient quantity. It is a powerful
hemolytic agent, and victims may have delayed
symptoms for up to 24 hours. Both chronic and
acute exposures to arsine are dangerous. Effects
of a single (acute) inhalation exposure include
rapid intravascular hemolysis, hemoglobinuria
with accompanying dark urine, malaise, dizziness,
headache, nausea, vomiting, abdominal
pain, diarrhea, and collapse. Pulmonary edema
may occur following overexposure. There may
be a delay of several hours before the onset of
signs or symptoms.
Effects of repeated (chronic) overexposure
may result in peripheral neuropathy, hyperpigmentation,
keratosis, cardiovascular disease,
and progressive anemia. Other effects of overexposure
include pulmonary edema, jaundice,
and severe hemolytic anemia. Severe kidney
damage may occur with oliguria or anuria leading
to uremia and death. Severe liver and cardiac
damage may also occur. There is evidence
(published in reports of the National Toxicology
Program and the International Agency for Research
on Cancer) that inorganic arsenic compounds
are skin and lung carcinogens in humans
First aid
If a person breathes in large amounts of thischemical, move the exposed person to fresh air at once andperform artificial respiration. If this chemical has beeninhaled, remove from exposure, begin rescue breathing(using universal precautions, including resuscitationmask) if breathing has stopped and CPR if heart actionhas stopped. Transfer promptly to a medical facility.Dimercaprol treatment is indicated and blood transfusionsmay be necessary. If this chemical gets into the eyes, removeany contact lenses at once and irrigate immediately for atleast 15 min, occasionally lifting upper and lower lids. Seekmedical attention immediately. If frostbite has occurred,seek medical attention immediately; do NOT rub theaffected areas or flush them with water. In order to preventfurther tissue damage, do NOT attempt to remove frozenclothing from frostbitten areas. If frostbite has NOToccurred, immediately and thoroughly wash contaminatedskin with soap and water. Seek medical attention immediately. Medical observation is recommended for 24°48 hafter breathing overexposure, as pulmonary edema may bedelayed. As first aid for pulmonary edema, a doctor orauthorized paramedic may consider administering a corticosteroid spray.Note to physician: For severe poisoning BAL [BritishAnti-Lewisite, Dimercaprol, dithiopropanol (C3H8OS2)]has been used to treat toxic symptoms of certain heavymetals poisoning including arsenic. Although BAL isreported to have a large margin of safety, caution must beexercised, because toxic effects may be caused by excessive dosage. Most can be prevented by premedication with1-ephedrine sulfate (CAS: 134-72-5). For milder poisoningpenicillamine (not penicillin) has been used, both withmixed success. Side effects occur with such treatment andit is never a substitute for controlling exposure. It can onlybe done under strict medical care.
Carcinogenicity
Arsenic has been considered a human carcinogen for a number of years(1),but the mechanisms underlying these processes have remained elusive due in part to the absence of an appropriate animal model. There are a number of hypotheses for the mechanisms of arsenical action that include arsenical inhibition of DNA repair, cocarcinogenesis, and more recently the concept of arsenical production of ROS(65,66) that may act in concert with these mechanisms. It is clear from in vitro mutagenicity test systems that arsenicals are not direct-acting mutagens but rather act via some secondary mechanism(s). Given the long history and knowledge that arsenicals in air and water produce human cancers, this is a remarkable situation with regard to occupational and environmental exposures. Most studies of animals exposed to arsenate or arsenite by the oral route have not detected any clear evidence for an increased incidence of skin cancer or other cancers. Recently, a series of studies presented evidence that inorganic arsenic may be a transplacental carcinogen in animals. Waalkes et al. exposed timed pregnant mice to sodium arsenite in drinking water during gestation days 8–18. Dose-related increases in hepatocellular carcinomas and adrenal tumors in the male offspring and uterine hyperplasia in female offspring from treated dams were reported. The offspring also had increase in the number of malignant tumors. Aberrant estrogen signaling, potentially through inappropriate estrogen receptora, may play a role in the arsenic-induced tumors in these offspring.
Environmental Fate
Arsine acts predominantly as a hemolytic agent. Hemolysis appears to be dependent on membrane disruption as a result of arsine reactions with sulfhydryl groups and from formation of hydrogen peroxide and adducts with oxyhemoglobin. Failure of the kidneys and other organs is probably not only due to the effects of red blood cell debris slugging within the microcirculation but also to a direct toxic effect on the organs.
Storage
cylinders of arsine should be stored and used in a continuously ventilated gas cabinet or fume hood. Local fire codes should be reviewed for limitations on quantity and storage requirements. Carbon steel, stainless steel, Monel?, and Hastelloy ?C are preferred materials for handling arsine; brass and aluminum should be avoided. Kel-F? and Teflon? are preferred gasket materials; Viton? and Nylon? are acceptable.
Shipping
UN2188 Arsine, Hazard class: 2.3; Labels: 2.3- Poisonous gas, 2.1-Flammable gas, Inhalation Hazard Zone A. Cylinders must be transported in a secure upright position, in a well-ventilated truck. Protect cylinder and labels from physical damage. The owner of the compressed gas cylinder is the only entity allowed by federal law (49CFR) to transport and refill them. It is a violation of transportation regulations to refill compressed gas cylinders without the express written permission of the owner. Military driver shall be given full and complete information regarding shipment and conditions in case of emergency. AR 50-6 deals specifically with the shipment of chemical agents. Shipments of agent will be escorted in accordance with AR 740-32.
Toxicity evaluation
Arsine is relatively stable in air and may travel in the atmosphere from a point of emission to remote areas, especially at night before it is converted into nonvolatile oxidized compounds, which may be further removed by particulate matter or deposited back to the soil. Since arsine is decomposed by the action of ultraviolet rays, its stability is reduced during the day. Arsine is moderately soluble in water (20 ml per 100 ml at 20°C). After entering the water, arsine is likely to be oxidized to other arsenic compounds, of which only a small percentage remain in the water; the rest are deployed along the zone of sediment, where they can undergo biomethylation by microorganisms.
Incompatibilities
Arsine forms explosive mixture with air. SA reacts with strong oxidizers, nitric acid, causing an explosion hazard. Thermally unstable; shock, friction, and concussion sensitive; can explosively decompose. Can explode on contact with warm, dry air. Violent reaction with acids, halogens, mixtures of potassium and ammonia. Decomposes to metallic arsenic (fumes) on exposure to light, moisture or upon decomposition from heat or ignition.
Waste Disposal
Return refillable compressed gas cylinders to supplier. Arsine may be disposed of by controlled burning. When possible, cylinders should be sealed and returned to suppliers. Seek guidance from regulatory agencies as to proper disposal. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal.
Precautions
Occupational workers should be careful during handling/use of arsine. Workers should use protective gloves: neoprene, butyl rubber, PVC, polyethylene, or Tefl on. Workers should also use appropriate protective equipment. If a leak occurs in a user’s equipment, be certain to purge the piping with an inert gas prior to attempting repairs and evacuate all personnel from the affected area. The compressed gas cylinders should not be refi lled without the express written permission of the owner.
GRADES AVAILABLE
Arsine is usually sold in ultrahigh-purity grades
(99.9999 percent) primarily for use in the electronic
industry.
Manufacturers supply various grades of product
depending on the application and purity
requirements such as:
? Electronic Grade
? Ultra Large Scale Integration (ULSI)
Grade
? Metal Organic Chemical Vapor Deposition
(MOCVD) Grade
? Semiconductor Grade
? MegaBit Grade
Properties of ARSINE
| Melting point: | -117° |
| Boiling point: | bp -62.5° |
| Density | 1.321 (estimate) |
| vapor pressure | >760 mmHg at 20 °C |
| solubility | slightly soluble in H2O |
| form | colorless gas |
| color | colorless |
| Odor | Garlic-like odor detectable at 0.5 to 1 ppm |
| Water Solubility | mL/100g H2O (760mm): 42 (0°C), 30 (10°C), 28 (20°C) [LAN05] |
| Exposure limits | TLV-TWA 0.2 mg/m3 (0.05 ppm) (ACGIH
and OSHA); 0.002 mg(As)/m3/15 min; ceiling
0.005 ppm(As)/15 min (NIOSH). |
| Dielectric constant | 2.5(-100℃) |
| Stability: | Stable, but pyrophoric. Capable of detonation with some initiators. Flammable. Incompatible with oxidizing agents, chlorine, nitric acid. |
| EPA Substance Registry System | Arsine (7784-42-1) |
Safety information for ARSINE
Computed Descriptors for ARSINE
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 1-methylindoline-2,3-dione 98%View Details
1-methylindoline-2,3-dione 98%View Details
2058-74-4 -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
