ARSENIC (III) SULFIDE
- CAS NO.:1303-33-9
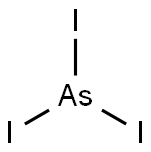
- Empirical Formula: As2S3
- Molecular Weight: 246.04
- MDL number: MFCD00003435
- EINECS: 215-117-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
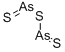
What is ARSENIC (III) SULFIDE?
Description
Arsenic trisulfide is a noncombustible, odorless, yellow or orange powder or red needles (changes to adifferent “red” form at 170℃). Molecular weight= 246.04;Specific gravity: 3.43 at 20℃; Boiling point= 707℃;Freezing/Melting point= 300°327℃. Hazard Identification(based on NFPA-704 M Rating System): Health 3,Flammability 2, Reactivity 0. Insoluble in water.
Chemical properties
Orpiment, As2S3, deep orange crystals to over 1 cm in a small group
Chemical properties
Arsenic trisulfide is a noncombustible, odorless, yellow or orange powder or red needles (changes to a different “red” form @ 170 C).
Physical properties
Yellow or orange monoclinic crystal or powder; a red allotrope modification also known; density 3.46 g/cm3; melts at 310°C; boils at 707°C; insoluble in water; soluble in liquid ammonia and alkalies.
Occurrence
Arsenic sesquisulfide occurs in nature as the mineral orpiment. It is used as a pigment; in the manufacture of infrared-transmitting glass; in semiconductors and photoconductors; in pyrotechnics; in linoleum and oil cloth; for the removal of hairs from hides; and as a reducing agent.
The Uses of ARSENIC (III) SULFIDE
manufacture of glass, particularly infrared-transmitting glass; manufacture of oil cloth, linoleum; in electrical semiconductors, photoconductors; as pigment; for depilating hides; in pyrotechnics.
The Uses of ARSENIC (III) SULFIDE
Arsenic(III) sulfide is used in the treatment of acute promyelocytic leukemia. It is an inorganic photoresist used in the fabrication of photonic crystals and 3-D nanostructures. It is also used as an acousto-optic material, pigment, tanning agent and chalcogenide glass for infrared optics. Further, it is used as an analytical test for presence of dissimilatory arsenic-reducing bacteria (DARB).
Preparation
Arsenic sesquioxide may be prepared by heating arsenic trioxide with hydrogen sulfide:
As2O3 + 3 H2S → As2S3 + 3 H2O
Alternatively, it may be precipitated out from a solution of arsenous acid or arsenic trioxide in dilute hydrochloric acid by passing hydrogen sulfide into the solution:
2H3AsO3 + 3H2S → As2S3 + 6H2O.
Definition
Arsenic(III) sulfide (Arsenic tri-sulfide glass) is a glassy material. It is important as the window of a cell for a sample containing water.
General Description
A yellow or red crystalline solid or powder. Combustible. Insoluble in water. Toxic by inhalation (dust) and ingestion.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
ARSENIC (III) SULFIDE is dissolved by alkalis and by hydrochloric acid (slowly). Decomposed by nitric acid. May react with oxidizing agents to generate heat and products that may be flammable, combustible, or otherwise reactive. The reactions may be violent. ARSENIC (III) SULFIDE reacts with concentrated solutions of chloric acid with incandescence [Mellor Supp. II Part I:584 1956]. Generates toxic gaseous hydrogen sulfide on contact with acids.
Health Hazard
(Acute and sub-acute poisoning are not common.) Repeated inhalation causes irritation of nose, laryngitis, mild bronchitis. Ingestion causes weakness, loss of appetite, gastrointestinal disturbances, peripheral neuritis, occasional hepatitis. Contact with eyes causes irritation. Irritates skin, especially where moist; if not treated, may cause ulceration.
Fire Hazard
Combustible material: may burn but does not ignite readily. Containers may explode when heated. Runoff may pollute waterways. Substance may be transported in a molten form.
Industrial uses
As2S3 (orpiment) and As2S2 (realgar) have been used as early as 2000 BC as drugs, for example, to cure cancerous tumours, ulcers and other diseases of the time. Later, Galen (130 200 AD) recommended the application of a paste of arsenic sulfide against ulcers.
Safety Profile
Confirmed human carcinogen with experimental tumorigenic data. A poison. Reacts violently with H202, (m03 + S). When heated to decomposition or on contact with acid or acid fumes it emits highly toxic fumes of S02, H2S, and As. Reacts with water or steam to emit toxic and flammable vapors.
Toxicology
Arsenic trisulfide is obtained by fusing arsenic trioxide with sulfur is marketed as orpiment. Or piment always contains unchanged arsenic tri oxide and, unlike the pure sulfide, is poisonous.
Potential Exposure
Arsenic trisulfide is used in the manufacture of glass, oil cloth, linoleum, electrical semiconductors, fireworks and used as a pigment.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit. Note to physician: For severe poisoning BAL [British AntiLewisite, Dimercaprol, dithiopropanol (C3H8OS2)] has beenused to treat toxic symptoms of certain heavy metals poisoning including arsenic. Although BAL is reported to have alarge margin of safety, caution must be exercised, becausetoxic effects may be caused by excessive dosage. Most canbe prevented by premedication with 1-ephedrine sulfate(CAS: 134-72-5). For milder poisoning penicillamine (notpenicillin) has been used, both with mixed success. Sideeffects occur with such treatment and it is never a substitutefor controlling exposure. It can only be done under strictmedical care.
storage
Color Code—Blue: Health Hazard/Poison: Store ina secure poison location. Prior to working with this chemicalyou should be trained on its proper handling and storage.Avoid contact with the incompatible materials cited above.A regulated, marked area should be established where thischemical is handled, used, or stored in compliance withOSHA Standard 1910.1045.
Shipping
UN1557 Arsenic compounds, solid, n.o.s. inorganic, including arsenates, n.o.s.; arsenites, n.o.s.; arsenic sulfides, n.o.s.; and organic compounds of arsenic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Incompatibilities
A strong reducing agent; avoid contact with oxidizers (such as perchlorates, peroxides, permanganates, chlorates, and nitrates) and potassium nitrate mixed with sulfur, since violent reactions occur. Water contact forms hydrogen sulfide. Incompatible with acids, halogens. Contact with acids or acid mists releases deadly arsine gas.
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal.
Properties of ARSENIC (III) SULFIDE
| Melting point: | 300 °C(lit.) |
| Boiling point: | 707 °C |
| Density | 3.43 g/mL at 25 °C(lit.) |
| Flash point: | 707°C |
| storage temp. | Poison room |
| solubility | insoluble in H2O; soluble in alkaline solutions |
| form | Powder |
| Specific Gravity | 3.43 |
| color | Yellow to orange |
| Water Solubility | Insoluble in water. Soluble in alkali sulfides or carbonate, hot HCl |
| Sensitive | moisture sensitive |
| Merck | 14,806 |
| Solubility Product Constant (Ksp) | pKsp: 21.68 |
| Exposure limits | ACGIH: TWA 0.01 mg/m3 NIOSH: IDLH 5 mg/m3; Ceiling 0.002 mg/m3 |
| CAS DataBase Reference | 1303-33-9(CAS DataBase Reference) |
| EPA Substance Registry System | Arsenic trisulfide (1303-33-9) |
Safety information for ARSENIC (III) SULFIDE
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H311:Acute toxicity,dermal H331:Acute toxicity,inhalation H400:Hazardous to the aquatic environment, acute hazard H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P270:Do not eat, drink or smoke when using this product. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P309:IF exposed or if you feel unwell: P310:Immediately call a POISON CENTER or doctor/physician. P311:Call a POISON CENTER or doctor/physician. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P302+P352:IF ON SKIN: wash with plenty of soap and water. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for ARSENIC (III) SULFIDE
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde Electrolytic Iron Powder 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 4-HYDROXY BENZYL ALCOHOL 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) S-2-CHLORO PROPIONIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 3-(Hydroxymethyl)benzoate N-Boc-2-chloroethylamine 1-Bromo-2-methoxy-3-nitrobenzene N-Methyl-3-cyclopenten-1-amine 2-Bromo-3-hydroxybenzaldehyde 1H-indazole-5-carboxamideRelated products of tetrahydrofuran
You may like
-
 Arsenic(III) sulfide CAS 1303-33-9View Details
Arsenic(III) sulfide CAS 1303-33-9View Details
1303-33-9 -
 Arsenic(III) sulfide CAS 1303-33-9View Details
Arsenic(III) sulfide CAS 1303-33-9View Details
1303-33-9 -
 Arsenic(III) sulfide CAS 1303-33-9View Details
Arsenic(III) sulfide CAS 1303-33-9View Details
1303-33-9 -
 Arsenic(III) sulfide CAS 1303-33-9View Details
Arsenic(III) sulfide CAS 1303-33-9View Details
1303-33-9 -
 Arsenic sulphide CAS 1303-33-9View Details
Arsenic sulphide CAS 1303-33-9View Details
1303-33-9 -
 1260741-78-3 6-Bromo-3-iodo-1-methyl-1H-indazole 98%View Details
1260741-78-3 6-Bromo-3-iodo-1-methyl-1H-indazole 98%View Details
1260741-78-3 -
 2490430-37-8 98%View Details
2490430-37-8 98%View Details
2490430-37-8 -
 N-(5-Amino-2-methylphenyl)acetamide 5434-30-0 98%View Details
N-(5-Amino-2-methylphenyl)acetamide 5434-30-0 98%View Details
5434-30-0