ARANIDIPINE
- CAS NO.:86780-90-7
- Empirical Formula: C19H20N2O7
- Molecular Weight: 388.37
- MDL number: MFCD00865813
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
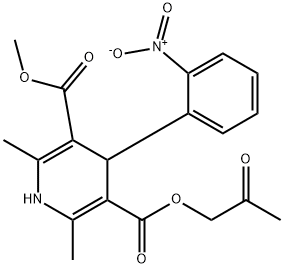
What is ARANIDIPINE?
Description
Aranidipine was launched in Japan as an antihypertensive agent. Its pharmacological effects are similar to other dihydropyridine derivatives, e.g., nefidipine, however, it is more potent and longer lasting. This is partially due to the fact that its initial metabolite (ketone reduction) is just as effective as the parent compound. Aranidipine exerts its activity by blocking Ca+2 entry during depolarization via L-type voltagegated Ca channels. This causes decreased levels of intracellular Ca which leads to enhanced relaxation of smooth and cardiac muscle. It is a selective α2-adrenoreceptor antagonist which inhibits vasoconstrictive responses. As a dihydropyridine derivative, it can be synthesized via a modified Hantsch synthesis. While sold as a racemate, the (S)-enantiomer is 150 times more active than the (R)- antipode.
Originator
Maruko Seiyaku (Japan)
Definition
ChEBI: Aranidipine is an organic molecular entity.
Manufacturing Process
100.0 mg of 50% sodium hydride was added to a mixture of 20.0 g of 2,2-
ethylenedioxypropanol and 100 ml of benzene, and 20.0 g of diketene was
added dropwise to the mixture while refluxing the mixture. After refluxing the
mixture for 2 h, the solvent was distilled off and the resulting residue was
distilled under reduced pressure to obtain 21.5 g (70% yield) of 2,2-
ethylenedioxypropyl acetoacetate as a colorless oil, boiling point of 90°C (6
mm Hg).
Ammonia gas was passed through a mixture of 19.0 g of 2,2-
ethylenedioxypropyl acetoacetate and 100 ml of methanol for 2.5 h under icecooling
while stirring. The solvent was then distilled off and the residue was
distilled under reduced pressure to obtain 16.0 g (84% yield) of 2,2-
ethylenedioxypropyl 3-aminocrotonate as a pale yellow oil, boiling point of
120°C (5 mm Hg).
A mixture of methyl 2'-nitrobenzylidene acetoacetate, 2,2-ethylenedioxypropyl
3-aminocrotonate and ethanol was refluxed for 10 h. The resulting reaction
solution was allowed to stand overnight, and the precipitated crystals were
collected by filtration and recrystallized from ethanol to obtain methyl 2,2-
ethylenedioxypropyl 2,6-dimethyl-4-(2-nitrophenyl)-1,4-dihydropyridine-3,5-
dicarboxylate.
Methyl 2,2-ethylenedioxypropyl 2,6-dimethyl-4-(2-nitrophenyl)-1,4-
dihydropyridine-3,5-dicarboxylate was refluxed in an ethanol solution
containing 10% hydrochloric acid for 6 h. The solvent was then distilled off
and the residue was crystallized from diethyl ether to give methyl 2-oxopropyl
2,6-dimethyl-4-(2-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate as
yellow prisms, melting point 155°C (recrystallized from a mixture of ethyl
acetate and hexane).
brand name
Bec/Sapresta
Therapeutic Function
Antihypertensive
Properties of ARANIDIPINE
| Melting point: | 155° |
| Boiling point: | 530.0±50.0 °C(Predicted) |
| Density | 1.284±0.06 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | DMSO : 125 mg/mL (321.86 mM) |
| form | Solid |
| pka | 2.56±0.70(Predicted) |
| color | Light yellow to yellow |
Safety information for ARANIDIPINE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P501:Dispose of contents/container to..… |
Computed Descriptors for ARANIDIPINE
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1,1’-CARBONYLDIIMIDAZOLE DIETHYL AMINOMALONATE HYDROCHLORIDE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8