Lacidipine
Synonym(s):3,5-Diethyl 4-{2-[(1E)-3-(tert-butoxy)-3-oxoprop-1-en-1-yl]phenyl}-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate;4-[2-[(1E)-3-(1,1-Dimethylethoxy)-3-oxo-1-propen-1-yl]phenyl]-1,4-dihydro-2,6-dimethyl-3,5-pyridinedicarboxylic acid 3,5-diethyl ester;CID 5311217;GR-43659X;GX-1048
- CAS NO.:103890-78-4
- Empirical Formula: C26H33NO6
- Molecular Weight: 455.54
- MDL number: MFCD00865936
- EINECS: 638-759-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-16 16:15:04
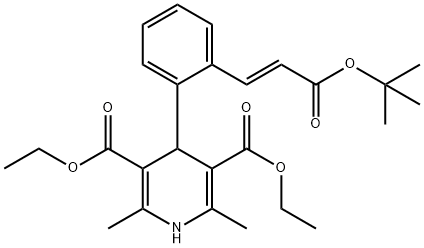
What is Lacidipine?
Absorption
Since it is a highly lipophilic compound, lacidpine is rapidly absorbed from the gastrointestinal tract following oral administration with the peak plasma concentrations reached between 30 and 150 minutes of dosing . The peak plasma concentrations display large interindividual variability, with the values ranging from 1.6 to 5.7 μg/L following single-dose oral administration of lacidipine 4mg in healthy young volunteers .
Absolute bioavailability is less than 10% due to extensive first-pass metabolism in the liver .
Toxicity
There have been no recorded cases of lacidipine tablets overdosage. Some of the symptoms of overdose include prolonged peripheral vasodilation associated with hypotension and
tachycardia. Bradycardia or prolonged AV conduction could theoretically occur. As there is no known antidote for lacidipine, the use of standard general measures for monitoring cardiac function and appropriate supportive and therapeutic measures is recommended .
Oral LD50 in mouse, rabbit and rat are 300mg/kg, 3200mg/kg and 980mg/kg, respectively .
Description
Lacidipine is a new second-generation dihydropyridine calcium antagonist introduced as a once a day treatment for mild to moderate hypertension. It is reported to have high selectivity for vascular smooth muscle and also a long duration of action. The use of lacidipine as an antiatherosclerotic agent is currently under investigation.
Description
Lacidipine is a dihydropyridine L-type calcium channel blocker. It induces relaxation of isolated rat aorta and inhibits calcium-induced contraction of rabbit ear artery (pA2 = 9.4). It also induces relaxation of calcium-induced contractions in isolated rat colon and bladder and guinea pig trachea (IC50s = 6.7, 6, and 7.8 nM, respectively). Lacidipine induces negative inotropy in isolated guinea pig ventricular strips (IC50 = 110 nM). It reduces mean blood pressure in spontaneously hypertensive rats (ED25 = 0.35 mg/kg) and in renal hypertensive dogs (ED25 = 0.22 mg/kg) with a transient increase in heart rate. Lacidipine inhibits copper-induced oxidation of isolated human LDL when used at concentrations of 1 and 5 μM. It reduces the extension of aortic atheromatous lesions and decreases renal injury in ApoE-/- mice in a model of Western diet-induced atherosclerosis.
Chemical properties
White-to-Off-White Crystalline Solid
Originator
Glaxo (United Kingdom)
The Uses of Lacidipine
A dihydropyridine calcium channel blocker. Antihypertensive
The Uses of Lacidipine
antihypertensive;dihydropyridinr calcium channel blocker
The Uses of Lacidipine
A dihydropyridine calcium channel blocker. Antihypertensive.
Background
Lacidipine is a lipophilic dihydropyridine calcium antagonist with an intrinsically slow onset of activity. Due to its long duration of action, lacidipine does not lead to reflex tachycardia . It displays specificity in the vascular smooth muscle, where it acts as an antihypertensive agent to dilate peripheral arterioles and reduce blood pressure. Compared to other dihydropyridine calcium antagonists, lacidipine exhibits a greater antioxidant activity which may confer potentially beneficial antiatherosclerotic effects . Lacidipine is a highly lipophilic molecule that interacts with the biological membranes. Through radiotracer analysis, it was determined that lacidipine displays a high membrane partition coefficient leading to accumulation of the drug in the membrane and slow rate of membrane washout . When visualized by small-angle X-ray diffraction with angstrom resolution to examine its location within the membranes, lacidipine was found deep within the membrane's hydrocarbon core . These results may explain the long clinical half-life of lacidipine .
In randomised, well-controlled trials, administration of daily single-dose lacidipine ranging from 2-6 mg demonstrated comparable antihypertensive efficacy similar to that of other long-acting dihydropyridine calcium antagonists, thiazide diuretics, atenolol (a beta-blocker) and enalapril (an ACE inhibitor) . It is available as once-daily oral tablets containing 2 or 4 mg of the active compound commonly marketed as Lacipil or Motens. It is not currently FDA-approved.
Indications
Indicated for the treatment of hypertension either alone or in combination with other antihypertensive agents, including β-adrenoceptor antagonists, diuretics, and ACE-inhibitors .
What are the applications of Application
trans Lacidipine is a calcium channel protein inhibitor and blocker
Definition
ChEBI: Lacidipine is a cinnamate ester and a tert-butyl ester.
brand name
Lacipil; Lacirex; Viapres
Biochem/physiol Actions
Lacidipine is a long-acting calcium antagonist that is used in the management of hypertension. Lacidipine is a L-type Ca(2+) channel blocker belonging to 1,4-dihydropyridine class. Also, Lacidipine inhibits ryanodine receptors on the ER membrane that enhances folding, trafficking and lysosomal activity of ERAD (ER-associated degradation) misfolded lysosomal glucocerebrosidase (GS).
Pharmacokinetics
acidipine is a specific and potent calcium antagonist with a predominant selectivity for calcium channels in the vascular smooth muscle. Its main action is to dilate predominantly peripheral and coronary arteries, reducing peripheral vascular resistance and lowering blood pressure .
Following the oral administration of 4 mg lacidipine to volunteer subjects, a minimal prolongation of QTc interval has been observed (mean QTcF increase between 3.44 and 9.60 ms in young and elderly volunteers) .
Metabolism
Lacidipine undergoes complete CYP3A4-mediated hepatic metabolism, with no parent drug detected in the urine or faeces. The 2 main metabolites have no pharmacological activity .
Properties of Lacidipine
| Melting point: | 174-175°C |
| Boiling point: | 558.4±50.0 °C(Predicted) |
| Density | 1.127±0.06 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | DMSO: soluble20mg/mL, clear |
| form | powder |
| pka | 3.00±0.70(Predicted) |
| color | white to beige |
| Merck | 14,5331 |
| CAS DataBase Reference | 103890-78-4(CAS DataBase Reference) |
Safety information for Lacidipine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P501:Dispose of contents/container to..… |
Computed Descriptors for Lacidipine
| InChIKey | GKQPCPXONLDCMU-CCEZHUSRSA-N |
Lacidipine manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran




You may like
-
 103890-78-4 Lacidipine 98%View Details
103890-78-4 Lacidipine 98%View Details
103890-78-4 -
 Lacidipine 98%View Details
Lacidipine 98%View Details
103890-78-4 -
 Lacidipine 98.00% CAS 103890-78-4View Details
Lacidipine 98.00% CAS 103890-78-4View Details
103890-78-4 -
 Lacidipine CAS 103890-78-4View Details
Lacidipine CAS 103890-78-4View Details
103890-78-4 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
