Azelnidipine
Synonym(s):2-Amino-1,4-dihydro-6-methyl-4-(3 nitrophenyl)-3,5-pyridinedicarboxylic acid 3-[1-(diphenylmethyl)-3-azetidinyl] 5-(1-methylethyl) ester;CS-905;RS-9054
- CAS NO.:123524-52-7
- Empirical Formula: C33H34N4O6
- Molecular Weight: 582.65
- MDL number: MFCD00865803
- EINECS: 634-143-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 20:33:22
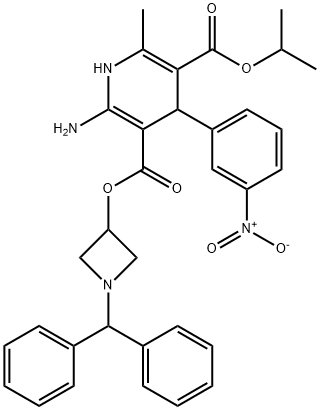
What is Azelnidipine?
Description
Azelnidipine, a member of the 1,4-dihydropyridine class of L-type calcium channel blockers with a slow onset profile, was marketed in Japan for the treatment of hypertension. Azelnidipine is synthesized via the condensation of iso-propyl 2-(3- nitrobenzylidene)acetoacetate with (1-diphenylmethylazetidin-3-yl)-3,3-diamino acrylate. The diamino acrylate intermediate is prepared from the cyanoacetic ester by sequential treatment with HCl and ammonia. In receptor binding studies using porcine heart membrane fractions, azelnidipine exhibits an IC50 of 3.1 nM and an apparent Ki of 2.1 nM. Its tight binding and slow onset are correlated with its high lipophilicity. A slow onset is also noted in vitro in a rat aortic strip contraction assay, and this effect continues after removal of the drug from the bath solution. In the conscious spontaneously hypertensive rat (SHR) model of hypertension, it was more potent that nicardipine and also had a more gradual onset and long-lasting effect. This effect was noted both when dosed orally or intravenously. When SHR dosed at 1 or 3 mpk/day for 15 weeks, a sustained reduction in systolic blood pressure was noted (19 and 43 mmHg reduction, respectively). Cardiac output was increased and total peripheral resistance was decreased in each group. Clinical studies of patients with mild-to-moderate hypertension have shown that long-term treatment with azelnidipine provided a sustained decrease in blood pressure (mean reduction systolic /diastolic: 27.8/16.6 mmHg). It similarly controlled blood pressure, as did amlodipine at 24 h. It possesses a gradual onset of activity with plasma levels increasing before the antihypotensive effect is attained. After plasma levels drop, the pharmacodynamic effect is sustained. In clinical studies, azelnidipine did not show reflex tachycardia, a common side effect of this class. Most common side effects were facial flushing and headache, similar to other dihydropyridines. Azelnidipine is dosed orally once daily (8–16 mg), is rapidly absorbed in a dose-dependent fashion, and has a mean terminal half-life of 19.2 h (8 mg dosage p.o. for seven days). Uniquely, it possesses a 2-amino function associated with a longer half-life than related agents wherein this moiety is a methyl. The very highly lipophilic 3-carboxylic ester side-chain is purported to contribute to the gradual onset of activity and prolonged pharmacodynamic effect, unlike other drugs in this class. This compound exhibits a much less pronounced first-pass metabolic effect than nicardipine.
Chemical properties
Yellow Solid
Originator
Sankyo (Japan)
The Uses of Azelnidipine
Azelnidipine is a dihydropyridine calcium channel blocker with antihypertensice activity. Azelnidipine is used for treating ischemic heart disease and cardiac remodeling after myocardial infarction. S tudies show that Azelnidipine ttreatment can reduce the risk of hyperglycemia induced metabolic disorders
What are the applications of Application
Azelnidipine is an L-type calcium channel protein inhibitor
What are the applications of Application
Azelnidipine is a novel dihydropyridine derivative, a L-type calcium channel blocker, and an antihypertensive. Acute administration of azelnidipine prevents a sudden drop of cardiac function after acute stress. Azelnidipine may have a protective role in inflammation associated with atherosclerosis.
Definition
ChEBI: Azelnidipine is an isopropyl ester.
brand name
Calblock
Biological Activity
Azelnidipine, a novel dihydropyridine derivative, is a L-type calcium channel blocker and antihypertensive. Unlike other L-type calcium channel blockers, azelnidipine causes minimal stimulation of the sympathetic nervous system despite its significant depressor effect. Azelnidipine may have a protective role in inflammation in atherosclerosis.
Synthesis
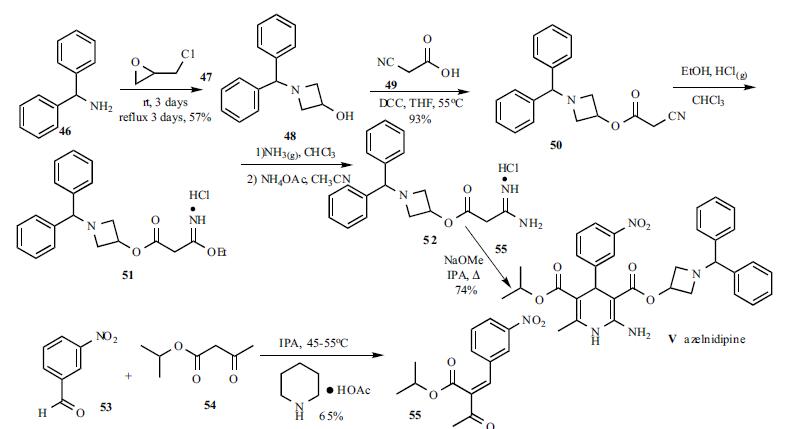
A solution of benzhydrylamine (46) and epichlorohydrin (47) was mixed without adding solvent to give azetidinol 48 in 57% yield. DCC coupling between cyanoacetic acid (49) and azetidinol 48 in hot THF gave ester 50 in 93% yield. Cyanoester 50 was treated with ethanol and HCl gas in chloroform to give imidate HCl salt 51, which was treated with ammonia gas in chloroform and ammonium acetate in acetonitrile to give the corresponding amidinoacetate 52. A modified Hantzsch reaction was employed to construct the 2-amino-1,4- dihydropyridine core structure. Compound 52 was condensed with 2-(3-nitrobenzylidene)acetic acid isopropyl ester (55) in the presence of NaOMe in refluxing isopropanol to give the cyclized product, azelnidipine (V) in 74% yield. Benzylideneacetoacetate 55 was obtained through the Knoevenagel reaction employing 3-nitrobenzaldehyde (53) and isopropyl acetoacetate (54) in isopropanol containing a catalytic amount of piperidinium acetate at 45-55oC in 65% yield.
Properties of Azelnidipine
| Melting point: | 120-126°C |
| Boiling point: | 709.3±60.0 °C(Predicted) |
| Density | 1.33±0.1 g/cm3(Predicted) |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| solubility | DMSO: >10mg/mL |
| pka | 7.89(at 25℃) |
| form | powder |
| color | Yellow |
| Merck | 14,907 |
| CAS DataBase Reference | 123524-52-7(CAS DataBase Reference) |
Safety information for Azelnidipine
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H318:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for Azelnidipine
| InChIKey | ZKFQEACEUNWPMT-UHFFFAOYSA-N |
Azelnidipine manufacturer
New Products
1-Boc-4-cyanopiperidine tert-Butyl carbazate 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE TETRABUTYLAMMONIUM CYANIDE TETRAHYDRO-2H-PYRAN-3-OL 3-Pyridineacrylic acid Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% Tadalafil Clopidogrel bisulfate Sitagliptin Phosphate Monohydrate Cabergoline Fexofinadine HCl Etoricoxib 4-Amino Acetophenone 2-Chloro Acetophenone Amlodipine Base 2,3,5-Triiodobenzoic Acid Pyrrolidine Diiodo PentoxideRelated products of tetrahydrofuran




You may like
-
 123524-52-7 Azelnidipine Reference Standard 98%View Details
123524-52-7 Azelnidipine Reference Standard 98%View Details
123524-52-7 -
 Azelnidipine 98%View Details
Azelnidipine 98%View Details -
 123524-52-7 Azelnidipine 99%View Details
123524-52-7 Azelnidipine 99%View Details
123524-52-7 -
 123524-52-7 98%View Details
123524-52-7 98%View Details
123524-52-7 -
 Azelnidipine 98%View Details
Azelnidipine 98%View Details
123524-52-7 -
 Azelnidipine 123524-52-7 98%View Details
Azelnidipine 123524-52-7 98%View Details
123524-52-7 -
 Azelnidipine CAS 123524-52-7View Details
Azelnidipine CAS 123524-52-7View Details
123524-52-7 -
 Azelnidipine CAS 123524-52-7View Details
Azelnidipine CAS 123524-52-7View Details
123524-52-7
