Amrinone
Synonym(s):5-Amino-(3,4′-bipyridin)-6(1H)-one;Inamrinone
- CAS NO.:60719-84-8
- Empirical Formula: C10H9N3O
- Molecular Weight: 187.2
- MDL number: MFCD00083228
- EINECS: 262-390-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 15:53:33
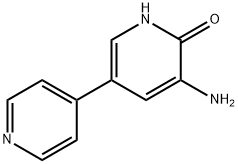
What is Amrinone?
Description
Amrinone is a positive inotropic agent useful in the management of severe congestive heart failure. It is effective even in unresponsive, fully digitalized patients.
Description
Amrinone is an inhibitor of phosphodiesterase 3 (PDE3; IC50 = 19.5 μM). It increases developed tension and contractile force in isolated cat papillary muscle. Amrinone (1-10 mg/kg) has positive inotropic effects, increasing the rate and force of heart contraction in anesthetized and unanesthetized dogs.
Chemical properties
Crystalline Solid
Originator
Sterling-Winthrop (USA)
The Uses of Amrinone
Amrinone is used for short-term treatment of cardiac insufficiency that does not respond to treatment of other drugs.
The Uses of Amrinone
A selective cAMP phosphodiesterase (PDE-3) inhibitor with positive inotropic and vasodilatory activity. Cardiotonic
The Uses of Amrinone
analgesic, antipyretic, antiinflammatory
Indications
Used in the treatment of congestive heart failure.
Background
Amrinone (or inamrinone) is a type 3 pyridine phosphodiesterase inhibitor. It is used in the treatment of congestive heart failure.
What are the applications of Application
Amrinone is a selective cAMP phosphodiesterase inhibitor
Definition
ChEBI: Amrinone is a 3,4'-bipyridine substituted at positions 5 and 6 by an amino group and a keto function respectively. A pyridine phosphodiesterase 3 inhibitor, it is a drug that may improve the prognosis in patients with congestive heart failure. It has a role as an EC 3.1.4.* (phosphoric diester hydrolase) inhibitor.
Manufacturing Process
A mixture containing 10g of 3-nitro-5-(4-pyridinyl)-2(1H)-pyridinone, 200 ml of dimethylformamide and 1.5 g of 10% palladium-on-charcoal was hydrogenated under pressure (50 psi) at room temperature until the uptake of hydrogen ceased (about 30 minutes). The reaction mixture was filtered through infusorial earth and the filtrate was heated in vacuum to remove the solvent. The residual material was crystallized from dimethylformamide, washed successively with ethanol and ether, and dried in a vacuum oven at 80°C for 8 hours to yield 6 g of 3-amino-5-(4-pyridinyl)-2(1H)-pyridinone, melting point 294° to 297°C with decomposition.
brand name
Inocor (Sterling Winthrop).
Therapeutic Function
Cardiotonic
General Description
During normal heart function, cAMP performsimportant roles in regulating intracellular calcium levels.That is, certain calcium channels and storage sites forcalcium must be activated by cAMP-dependant protein kinases.Because cAMP plays an indirect role in the contractilityprocess, agents that inhibit its degradation will providemore calcium for cardiac contraction. One phosphodiesteraseenzyme that is involved in the hydrolysis of myocardiumcAMP is F-III. Amrinone, 5-amino (3,4 -dipyridin)-6 1 (H)-one (Inocor), possesses positive isotropic effects as aresult of its ability to inhibit this phosphodiesterase. In 1999,the USP Nomenclature Committee and the United StatesAdopted Names (USAN) Council approved changing thenonproprietary name and the current official monograph titleof amrinone to inamrinone. This change in nomenclature wasa result of amrinone being confused with amiodarone becauseof the similarity of the names. This was reported to cause confusionbetween the products that led to medication errors,some of which resulted in serious injury or death.
Pharmacokinetics
Amrinone is a positive inotropic cardiotonic with vasodilator properties, phosphodiesterase inhibitory activity, and the ability to stimulate calcium ion influx into the cardiac cell.
Safety Profile
Poison by ingestion andintravenous routes. Human systemic effects by ingestion:cardiac arrhythmias, liver function, thrombocytopenia. Anexperimental teratogen. Other experimental reproductiveeffects. When heated to decomposition it emits toxicfumes o
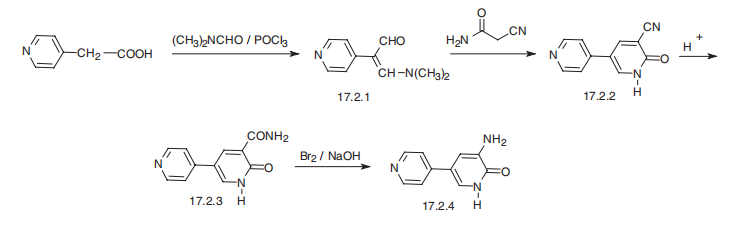
Synthesis
Amrinone, 3-amino-5-(4-piridinyl)-2(1H)-pyridinone (17.2.4), can be synthesized
from piridine-4-acetic acid, the reaction of which with a mixture of diemthylformamide?a
phosphorous oxychloride gives 2-(4-piridyl)-3-dimethylaminoacrolein (17.2.1).
Reacting this with cyanoacetamide gives 3-cyano-5-(4-piridyl)-2(1H)-pyidinone (17.2.2).
Hydrolysis of the cyano group of this product gives 3-carbamyl-5-(4-piridyl)-2(1H)-pyidinone
(17.2.3). A Hofmann rearrangement of this product (using bromine in sodium
hydroxide) gives amrinone (17.2.4).
An alternative method for the synthesis of amrinone from 3-cyano-5-(4-piridyl)-2(1H)-
pyridinone (17.2.2) is based on it?ˉs acidic hydrolysis to the corresponding acid, 3-carboxy-
5-(4-piridyl)-2(1H)-pyridinone (17.2.5), nitration of which with nitrous acid in the presence
of sulfuric acid forms 3-nitro-5-(4-piridyl)-2(1H)-pyridinone (17.2.6). Reducing the nitro
group of this product with hydrogen gives the desired amrinone (17.2.4).
Metabolism
Hepatic.
Properties of Amrinone
| Melting point: | 294-297°C (dec.) |
| Boiling point: | 321.94°C (rough estimate) |
| Density | 1.2025 (rough estimate) |
| refractive index | 1.4830 (estimate) |
| storage temp. | Keep in dark place,Sealed in dry,2-8°C |
| solubility | DMF: 0.3 mg/ml; DMSO: 0.5 mg/ml |
| pka | 12.05±0.10(Predicted) |
| form | Crystals |
| color | Crystals from DMF |
| Water Solubility | Insoluble in water or chloroform. Slightly soluble in acetic acid or DMF. Soluble in DMSO |
| CAS DataBase Reference | 60719-84-8(CAS DataBase Reference) |
Safety information for Amrinone
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral |
Computed Descriptors for Amrinone
Amrinone manufacturer
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
 Amrinone CAS 60719-84-8View Details
Amrinone CAS 60719-84-8View Details
60719-84-8 -
 Amrinone 98% (HPLC) CAS 60719-84-8View Details
Amrinone 98% (HPLC) CAS 60719-84-8View Details
60719-84-8 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8