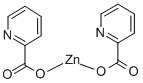
Zinc pyrithione
Synonym(s):Mercaptopyridine N-oxide zinc salt;Pyrithione
- CAS NO.:13463-41-7
- Empirical Formula: C10H8N2O2S2Zn
- Molecular Weight: 317.7
- MDL number: MFCD00067336
- EINECS: 236-671-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30

What is Zinc pyrithione?
Description
Zinc pyrithione is the zinc complex of 1-hydroxy-2(1H)-pyridinethione, or more commonly, pyrithione. Pyrithione was first?synthesized?by E. Shaw and co-workers in 1950. The preparation of the zinc derivative was disclosed by Olin Mathieson in a 1956 British patent, but the compound has been used since the 1930s.
Zinc pyrithione was originally used as an antibacterial and antifungal agent. It is still used as an antifungal, primarily as a treatment for dandruff and seborrheic dermatitis. Its current antibacterial uses are treating?Streptococcus?and?Staphylococcus?infections and psoriasis, eczema, ringworm, and athlete’s foot. Its primary nonmedical use is as a component in exterior paints to protect against mildew and algae.
?
Description
Zinc pyrithione is a coordination complex of zinc and pyrithione that has antimicrobial and anticancer activities. It is active against the bacteria E. coli, S. aureus, K. pneumoniae, A. baumannii, P. aeruginosa, E. faecium, E. faecalis, and E. cloacae (MICs = 1-4 μg/ml) and the fungus P. ovale when used at concentrations ranging from 0.01 to 10 μg/ml. Zinc pyrithione reduces tumor growth in an SCC-4 mouse xenograft model when administered at a dose of 1 mg/kg per week for six weeks. Formulations containing zinc pyrithione have been used in the treatment of dandruff.
Chemical properties
Beige granules
The Uses of Zinc pyrithione
Zinc pyrithione is directly cytotoxic and has antimicrobial effects. Zinc pyrithione is found in many shampoos (DHS-Zinc, Head and Shoulders) and should be applied for 5 minutes daily for 2 weeks.
Zinc pyrithione is the active ingredient in several shampoos used to control dandruff and seborrheic dermatitis and is also effective in the therapy of tinea versicolor. It remains unclear whether the beneficial effects are caused by an antiproliferative or antimicrobial effect or both. It is substantive to the hair, allowing continued therapeutic effect after washing.
The Uses of Zinc pyrithione
zinc pyrithione is a preservative against bacteria, fungi, and yeast. It is unstable in light and in the presence of oxidizing agents. Zinc pyrithione is useful in gels, creams, heavy lotions, and talcum powder.
The Uses of Zinc pyrithione
Reactions may lead to photosensitive eczema and actinic reticuloid syndrome. Zinc pyrithione is used as antifungal, antibacterial and antiseborrheic agent used in many shampoos and hair creams.
Indications
Zinc pyrithione is the active ingredient in several shampoos used to control dandruff and seborrheic dermatitis and is also effective in the therapy of tinea versicolor. It remains unclear whether the beneficial effects are caused by an antiproliferative or antimicrobial effect or both. It is substantive to the hair, allowing continued therapeutic effect after washing.
What are the applications of Application
1-Hydroxypyridine-2-thione zinc salt is a useful building block for chemical synthesis
brand name
Head & Shoulders Conditioner (Procter & Gamble).
General Description
Fine beige granules.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Organometallics, such as Zinc pyrithione, are reactive with many other groups. Incompatible with acids and bases. Organometallics are good reducing agents and therefore incompatible with oxidizing agents. Often reactive with water to generate toxic or flammable gases.
Fire Hazard
Flash point data for Zinc pyrithione are not available, but Zinc pyrithione is probably combustible.
Contact allergens
Zinc pyrithione is widely used in antidandruff shampoos and is a classic allergen. Concomitant reactions are expected to both zinc and sodium pyrithione.
Safety Profile
Poison by ingestion, skin contact, intraperitoneal, and intravenous routes. Moderately toxic by subcutaneous route. An experimental teratogen. Experimental reproductive effects. An eye irritant. When heated to decomposition it emits very toxic fumes of NOx, SOx, and ZnO. Used as an anti- dandruff agent in shampoos. See also ZINC COMPOUNDS and SULFIDES.
Synthesis
1000 g of sodium pyrithione, 20% aqueous solution (1.34 mmol of sodium pyrithione) was added to a 2L reactor equipped with a mechanical agitator and warmed to 70 °C with stirring. To the solution was added 190 g of 50 % zinc chloride (0.7 mmol of zinc chloride) over 10 minutes. After the completion of the addition, the solution was stirred at 70 °C for one hour, dehydrated and then washed with 500 g of distilled water. The solid thus obtained was dried with a heated-air drier at 70 °C to have a moisture content of less than 0.5 % and ground with a hammer mill to obtain a find powder of zinc pyrithione. The zinc pyrithione thus obtained was 212 g with a yield of 99.5 %.
Properties of Zinc pyrithione
| Melting point: | approximate 240℃ |
| Density | 1.782 g/cm3(Temp: 25 °C) |
| vapor pressure | 0Pa at 25℃ |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | almost transparency in Pyridine |
| form | Powder |
| color | White to Orange to Green |
| Odor | Mild Characteristic |
| Water Solubility | Insoluble (<0.1 g/100 mL at 21 ºC) |
| Merck | 14,7994 |
| InChI | InChI=1S/2C5H5NOS.Zn/c2*7-6-4-2-1-3-5(6)8;/h2*1-4,8H;/q;;+2/p-2 |
| CAS DataBase Reference | 13463-41-7(CAS DataBase Reference) |
| EPA Substance Registry System | Zinc pyrithione (13463-41-7) |
Safety information for Zinc pyrithione
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H318:Serious eye damage/eye irritation H330:Acute toxicity,inhalation H372:Specific target organ toxicity, repeated exposure H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Zinc pyrithione
| InChIKey | OTPSWLRZXRHDNX-UHFFFAOYSA-L |
| SMILES | C1(C=CC=C[N+]=1[O-])S[Zn]SC1C=CC=C[N+]=1[O-] |
Zinc pyrithione manufacturer
Salicylates and Chemicals Pvt. Ltd.
Speciality Organics Pvt. Ltd.
Emmennar Pharma Pvt Ltd
Kumar Organic Products Ltd
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3,4-Dibenzyloxybenzaldehyde 4-Hydrazinobenzoic acid 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 4-HYDROXY BENZYL ALCOHOL 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 3-NITRO-2-METHYL ANILINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

![[2,2'-dithiobis[pyridine] 1,1'-dioxide-O,O',S][sulphato(2-)-O]magnesium](https://img.chemicalbook.in/CAS/20210111/GIF/43143-11-9.gif)

You may like
-
 13463-41-7 98%View Details
13463-41-7 98%View Details
13463-41-7 -
 Zinc pyrithione 98%View Details
Zinc pyrithione 98%View Details
13463-41-7 -
 ZINC PYRITHIONE CAS 13463-41-7View Details
ZINC PYRITHIONE CAS 13463-41-7View Details
13463-41-7 -
 Zinc pyrithione >98% CAS 13463-41-7View Details
Zinc pyrithione >98% CAS 13463-41-7View Details
13463-41-7 -
 2-Mercaptopyridine N-Oxide Zinc Salt CAS 13463-41-7View Details
2-Mercaptopyridine N-Oxide Zinc Salt CAS 13463-41-7View Details
13463-41-7 -
 Zinc pyrithione 13463-41-7 99%View Details
Zinc pyrithione 13463-41-7 99%View Details
13463-41-7 -
 Zinc Pyrithione CASView Details
Zinc Pyrithione CASView Details -
 1-Hydroxypyridine-2-thione zinc salt CAS 13463-41-7View Details
1-Hydroxypyridine-2-thione zinc salt CAS 13463-41-7View Details
13463-41-7