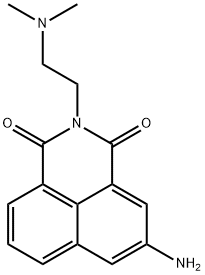
Amonafide
Synonym(s):5-Amino-2-[2-(dimethylamino)ethyl]-1H-benz[de]isoquinoline-1,3(2H)-dione;AS1413;FA 142;Nafidimide;NSC 308847
- CAS NO.:69408-81-7
- Empirical Formula: C16H17N3O2
- Molecular Weight: 283.33
- MDL number: MFCD00866438
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 20:33:22
What is Amonafide?
Chemical properties
Brownish Yellow Solid
The Uses of Amonafide
Amonafide is a DNA intercalator and topoisomerase II inhibitor in clinical development for the treatment of neoplastic diseases. Antitumor agent.
What are the applications of Application
Amonafide is a DNA intercalator and topoisomerase II inhibitor
Definition
ChEBI: 5-amino-2-[2-(dimethylamino)ethyl]benzo[de]isoquinoline-1,3-dione is a member of isoquinolines.
Hazard
A poison
Biological Activity
amonafide is a novel topoisomerase ii inhibitor. topoisomerase ii plays critical roles including dna transcription, replication and chromosome segregation. though the biological functions of topoisomerase ii are important for insuring genomic integrity, the ability to interfere with topoisomerase ii and generate enzyme mediated dna damage is an effective strategy for cancer chemotherapy.
in vitro
amonafide intercalated with dna and disrupted the loading of topoisomerases. in contrast to the classic agents, amonafide was found to induce higher molecular weight fragmentation, resulting in the apoptosis without dna cleavage. amonafide was found to act in an atp-independent manner and seemed unlikely to induce the chromosome translocations associated with treatmentinduced leukemia [1].
in vivo
amonafide was found to be able to inhibit ip l1210 leukemia, with optimal increased life spans (ils) of 61% to 106% following single 16 mg/kg dosing on days 1 to 9. similar efficacy was noted against ip p388 murine leukemia and the sc implanted l1210 leukemia. additionally, amonafide demonstrated activity against two nonleukemic ip implanted murine tumors, the m5076 sarcoma and the b16 melanoma [2].
References
[1] freeman cl,swords r,giles fj. amonafide: a future in treatment of resistant and secondary acute myeloid leukemia expert rev hematol.2012 feb;5(1):17-26.
[2] saez r,craig jb,kuhn jg,weiss gr,koeller j,phillips j,havlin k,harman g,hardy j,melink tj, et al. phase i clinical investigation of amonafide. j clin oncol.1989 sep;7(9):1351-8.
Properties of Amonafide
| Melting point: | 162-164°C |
| Boiling point: | 500.2±35.0 °C(Predicted) |
| Density | 1.306±0.06 g/cm3(Predicted) |
| storage temp. | Keep in dark place,Sealed in dry,2-8°C |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 8.83±0.28(Predicted) |
| color | Yellow to Dark Yellow |
Safety information for Amonafide
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Amonafide
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran





You may like
-
 Amonafide CAS 69408-81-7View Details
Amonafide CAS 69408-81-7View Details
69408-81-7 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8