Ammonium persulfate
Synonym(s):Ammonium Persulfate;APS;AP;Ammonium peroxydisulfate;Ammonium peroxodisulfate
- CAS NO.:7727-54-0
- Empirical Formula: H8N2O8S2
- Molecular Weight: 228.2
- MDL number: MFCD00003390
- EINECS: 231-786-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
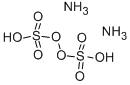
What is Ammonium persulfate?
Description
Persulfates are strong oxidizing agents widely used in the production of metals, textiles, photographs, cellophane, rubber, adhesive papers, foods, soaps, detergents and hair bleaches. Ammonium persulfate is used as a hair bleaching agent. It may induce irritant dermatitis, contact urticaria and allergic contact dermatitis and represents a major allergen in hairdressers.
Chemical properties
Off-white crystalline powder
Chemical properties
Ammonium persulfate is a colorless or white crystalline solid.
The Uses of Ammonium persulfate
Ammonium Persulfate is a bleaching agent for food starch that is used up to 0.075% and with sulfur dioxide up to 0.05%.
The Uses of Ammonium persulfate
Used for detection and determination of manganese and iron.
The Uses of Ammonium persulfate
As oxidizer and bleacher; to remove hypo; reducer and retarder in photography; in dyeing, manufacture of aniline dyes; oxidizer for copper; etching zinc; decolorizing and deodorizing oils; electroplating; washing infected yeast; removing pyrogallol stains; making soluble starch; depolarizer in electric batteries; In animal chemistry chiefly for detection and determination of manganese.
What are the applications of Application
Ammonium persulfate is APS catalyst for acrylamide gel polymerization and also used with TEMED to promote polymerization
General Description
A white crystalline solid. A strong oxidizing agent. Does not burn readily, but may cause spontaneous ignition of organic materials. Used as a bleaching agent and as a food preservative.
Air & Water Reactions
Soluble in water.
Reactivity Profile
Ammonium persulfate is a potent oxidizing agent. A powdered mixture with aluminum and water can explode [NFPA 491M 1991]. A mixture with sodium peroxide will explode if subjected to friction (crushing in a mortar), heating, or if a stream of carbon dioxide is passed over Ammonium persulfate [Mellor 10:464 1946-47]. Acidic solutions dissolve iron violently, [Mellor, 1947, Vol. 10, 470].
Hazard
Fire risk in contact with reducers.
Health Hazard
Inhalation produces slight toxic effects. Contact with dust irritates eyes and causes skin rash.
Flammability and Explosibility
Non flammable
Contact allergens
Persulfates are strong oxidizing agents widely used in the production of metals, textiles, photographs, cellophane, rubber, adhesive papers, foods, soaps, detergents, and hair bleaches. Ammonium persulfate is used as a hair bleaching agent. It may induce irritant dermatitis, (mainly) nonimmunologic contact urticaria, and allergic contact dermatitis and represents a major allergen in hairdressers. People reacting to ammonium persulfate also react to other persulfates such as potassium persulfate.
Safety Profile
Poison by intravenous and intraperitoneal routes. Moderately toxic by ingestion. A powerful oxidizer that can react vigorously with reducing agents. Releases oxygen when heated. Mxtures with sodium peroxide are explosives sensitive to friction, heating above 75℃, or contact with CO2 or water. Mixtures with (powdered aluminum + water) or (zinc + ammonia) are explosive. Violent reaction with iron or solutions of ammonia + silver salts. Solution with sulfuric acid is a strong oxidzing cleaning solution. When heated to decomposition it emits toxic fumes of SO,, NH3, and NOx.
Potential Exposure
It is used as a bleaching agent, in photographic chemicals, and to make dyes. It is also used as an ingredient of polymerization catalysts.
Shipping
UN1444 Ammonium persulfate, Hazard Class: 5.1; Labels: 5.1-Oxidizer
Purification Methods
Recrystallise it at room temperature from EtOH/water. It gradually loses NH3 on exposure to air. Its solubility is 0.5g/mL at 20o, and 2g/mL at 100o.
Incompatibilities
Decomposes in water and moist air, forming oxygen gas. A strong oxidizer; reacts with reducing agents; organic and combustible materials. Incompatible with heat, sodium peroxide (produces a friction-, heat-, and water-sensitive explosive); aluminum powder.
Waste Disposal
May be treated with large volumes of water, neutralized and flushed to sewer. This applies to small quantities only.
Properties of Ammonium persulfate
| Melting point: | 120 °C |
| Density | 1.98 |
| vapor density | 7.9 (vs air) |
| vapor pressure | 0Pa at 25℃ |
| refractive index | 1.50 |
| storage temp. | Store at +15°C to +25°C. |
| solubility | H2O: soluble |
| form | powder |
| color | White to yellow |
| Specific Gravity | 1.982 |
| PH Range | 1 - 2 |
| Odor | Odorless |
| PH | 1.0-2.5 (25℃, 1M in H2O) |
| Water Solubility | 582 g/L (20 ºC) decomposes |
| Sensitive | Moisture Sensitive |
| Merck | 14,541 |
| Exposure limits | ACGIH: TWA 0.1 mg/m3 |
| Stability: | Stable. Oxidizing. May ignite combustible material. Incompatible with bases, combustible material, hydrogen peroxide, peroxy compounds, silver compounds, zinc. May decompose upon exposure to water or moist air. |
| CAS DataBase Reference | 7727-54-0(CAS DataBase Reference) |
| EPA Substance Registry System | Ammonium peroxydisulfate (7727-54-0) |
Safety information for Ammonium persulfate
| Signal word | Danger |
| Pictogram(s) |
 Flame Over Circle Oxidizers GHS03  Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H272:Oxidising liquids;Oxidising solids H302:Acute toxicity,oral H315:Skin corrosion/irritation H317:Sensitisation, Skin H319:Serious eye damage/eye irritation H334:Sensitisation, respiratory H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Ammonium persulfate
| InChIKey | ROOXNKNUYICQNP-UHFFFAOYSA-N |
Ammonium persulfate manufacturer
JSK Chemicals
ARRAKIS INDUSTRIES LLP
Related products of tetrahydrofuran


You may like
-
 Ammonium persulfate 98%View Details
Ammonium persulfate 98%View Details -
 Ammonium peroxydisulfate CAS 7727-54-0View Details
Ammonium peroxydisulfate CAS 7727-54-0View Details
7727-54-0 -
 Ammonium Persulphate (APS) for molecular biology CAS 7727-54-0View Details
Ammonium Persulphate (APS) for molecular biology CAS 7727-54-0View Details
7727-54-0 -
 Ammonium Persulphate (APS) pure CAS 7727-54-0View Details
Ammonium Persulphate (APS) pure CAS 7727-54-0View Details
7727-54-0 -
 Ammonium Persulphate (APS) extrapure AR CAS 7727-54-0View Details
Ammonium Persulphate (APS) extrapure AR CAS 7727-54-0View Details
7727-54-0 -
 Ammonium Persulfate CASView Details
Ammonium Persulfate CASView Details -
 Ammonium persulfate 98% CAS 7727-54-0View Details
Ammonium persulfate 98% CAS 7727-54-0View Details
7727-54-0 -
 Ammonium peroxodisulfate CAS 7727-54-0View Details
Ammonium peroxodisulfate CAS 7727-54-0View Details
7727-54-0