Sodium persulfate
Synonym(s):Sodium peroxidisulfate;Sodium peroxodisulfate;Sodium persulfate, Peroxydisulfuric acid disodium salt
- CAS NO.:7775-27-1
- Empirical Formula: Na2O8S2
- Molecular Weight: 238.1
- MDL number: MFCD00003501
- EINECS: 231-892-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
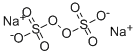
What is Sodium persulfate?
Chemical properties
White, crystalline powder. Soluble in water; decomposed by alcohol; decomposes in moist air.
The Uses of Sodium persulfate
Bleaching and oxidizing agent; promoter for emulsion polymerization reactions.
The Uses of Sodium persulfate
Sodium peroxydisulfate is used as a radical initiator for emulsion polymerization reactions like acrylonitrile butadiene styrene, detergent component, soil conditioner and soil remediation. It is also used for curing of formaldehyde adhesives. It acts as a bleaching agent and in the production of dyestuffs. It finds application in zinc and printed circuit boards and considered to be a replacement for ammonium persulfate in etching mixtures. Further, it is used in the preparation of diapocynin from apocynin. It plays a role in the conversion of phenols to para-diphenols in alkaline solution and of arylamines to aminophenols. It is actively involved in the Elbs persulfate oxidation and the Boyland-Sims oxidation reactions as an oxidizing agent.
The Uses of Sodium persulfate
Redi-Dri? is an innovative packaging product line that helps eliminate clumping material by adding extra packaging process steps without any chemical additives, anti-clumping agents, or hydrophobic compounds. Redi-Dri? offers a more convenient and safer way to handle the products.
What are the applications of Application
Sodium persulfate is a strongly oxidizing chemical used as a bleaching and oxidizing agent
General Description
A white crystalline solid. Very irritating to skin and eyes. May be toxic by skin absorption. Used as a bleaching agent.
Air & Water Reactions
Water soluble. Decomposes slowly in moist air.
Reactivity Profile
Sodium persulfate is a strong oxidizing agent. Reacts with many combustible materials and reducing agents, often vigorously enough to start fires or cause explosions [Handling Chemicals Safely 1980 p. 855]. Decomposes gradually under ordinary conditions decomposition is promoted by moisture and heat [Merck]. Decomposed by alcohol and silver ions [Merck].
Hazard
By ingestion, strong irritant to tissue.
Health Hazard
Inhalation, ingestion or contact (skin, eyes) with vapors or substance may cause severe injury, burns or death. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may cause pollution.
Fire Hazard
These substances will accelerate burning when involved in a fire. Some may decompose explosively when heated or involved in a fire. May explode from heat or contamination. Some will react explosively with hydrocarbons (fuels). May ignite combustibles (wood, paper, oil, clothing, etc.). Containers may explode when heated. Runoff may create fire or explosion hazard.
Flammability and Explosibility
Non flammable
Safety Profile
Poison by intraperitoneal and intravenous routes. A powerful oxidizer; can cause fires. When heated to decomposition it emits toxic fumes of SOx and Na2O. See also SULFATES.
Properties of Sodium persulfate
| Melting point: | 100 °C |
| Density | 2,4 g/cm3 |
| vapor pressure | 0Pa at 25℃ |
| storage temp. | Store at +15°C to +25°C. |
| solubility | H2O: 1 M at 20 °C, clear, colorless |
| form | Solid |
| color | White to yellow |
| Specific Gravity | 2.4 |
| PH Range | 2.5 - 4.0 |
| PH | 3.5-3.8 (100g/l, H2O, 20℃) |
| Odor | Odorless |
| Water Solubility | 550 g/L (20 ºC) |
| Merck | 14,8656 |
| Exposure limits | ACGIH: TWA 0.1 mg/m3 |
| Stability: | Stability Unstable. Strong oxidizer. Contact with combustible material may cause fire. Incompatible with combustible material, strong reducing agents, strong bases, alcohols, aluminium, magnesium. Protect from moisture. |
| CAS DataBase Reference | 7775-27-1(CAS DataBase Reference) |
| EPA Substance Registry System | Sodium persulfate (7775-27-1) |
Safety information for Sodium persulfate
| Signal word | Danger |
| Pictogram(s) |
 Flame Over Circle Oxidizers GHS03  Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H272:Oxidising liquids;Oxidising solids H302:Acute toxicity,oral H315:Skin corrosion/irritation H317:Sensitisation, Skin H334:Sensitisation, respiratory H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P220:Keep/Store away from clothing/…/combustible materials. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. |
Computed Descriptors for Sodium persulfate
| InChIKey | CHQMHPLRPQMAMX-UHFFFAOYSA-L |
Sodium persulfate manufacturer
Kronox Lab Sciences Pvt Ltd
Ralington Pharma
ARRAKIS INDUSTRIES LLP
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1,1’-CARBONYLDIIMIDAZOLE DIETHYL AMINOMALONATE HYDROCHLORIDE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
 Sodium persulfate 98%View Details
Sodium persulfate 98%View Details -
 Sodium peroxydisulfate CAS 7775-27-1View Details
Sodium peroxydisulfate CAS 7775-27-1View Details
7775-27-1 -
 Sodium Persulphate pure CAS 7775-27-1View Details
Sodium Persulphate pure CAS 7775-27-1View Details
7775-27-1 -
 Sodium persulphate, GR 99%+ CAS 7775-27-1View Details
Sodium persulphate, GR 99%+ CAS 7775-27-1View Details
7775-27-1 -
 Sodium persulphate CAS 7775-27-1View Details
Sodium persulphate CAS 7775-27-1View Details
7775-27-1 -
 Sodium Persulfate CASView Details
Sodium Persulfate CASView Details -
 Sodium persulfate CAS 7775-27-1View Details
Sodium persulfate CAS 7775-27-1View Details
7775-27-1 -
 Sodium Persulphate CASView Details
Sodium Persulphate CASView Details