Ammonium iron(II) sulfate
- CAS NO.:10045-89-3
- Empirical Formula: FeH5NO4S
- Molecular Weight: 170.95
- MDL number: MFCD00051039
- EINECS: 233-151-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02

What is Ammonium iron(II) sulfate?
Description
Ferrous ammonium sulfate is a pale green orblue-green solid (powder or lumpy crystals). Molecularweight = 392.14. Hazard Identification (based on NFPA-704 M Rating System): Health 3, Flammability 0,Reactivity 1. Soluble in water.
Chemical properties
Ferrous ammonium sulfate is a pale green or blue-green solid (powder or lumpy crystals).
Physical properties
Bluish-green monoclinic crystal; density 1.86 g/cm3; deomposes at 100°C;soluble in water; insoluble in alcohol.
The Uses of Ammonium iron(II) sulfate
Iron ammonium sulfate is an analytical reagent in titrations and colori-metric measurements to measure oxidizing substances, such as chlorine, or tomeasure the chemical oxygen demand in waste water. The compound is usedto prepare Fe(II) standard solution for these analyses. It also is a calibration standard in magnetic measurements; a reducing agent; a catalyst for poly-merization; and is used in photographic chemistry.
Definition
ChEBI: A compound of ammonium, iron and sulfate in which the ratio of ammonium to iron(2+) to sulfate ions is 2:1:2.
Preparation
Ferrous ammonium sulfate may be prepared by mixing an equimolar solution of ferrous sulfate and ammonium sulfate, followed by evaporation andcrystallization.
General Description
A light green crystalline solid. The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment. Ammonium iron(II) sulfate is used in medicine, chemical analysis and metallurgy.
Air & Water Reactions
Soluble in water that forms weakly acidic solutions. Deliquesces in air.
Reactivity Profile
Ammonium iron(II) sulfate is a weak reducing agent. Irritating and toxic ammonia and oxides of nitrogen may form in fires [USCG, 1999].
Health Hazard
Inhalation of dust irritates nose and throat. Ingestion causes irritation of mouth and stomach. Dust irritates eyes and can irritate skin on prolonged contact.
Fire Hazard
Special Hazards of Combustion Products: Irritating and toxic ammonia and oxides of nitrogen may form in fires.
Flammability and Explosibility
Not classified
Potential Exposure
This substance is used in photography, analytical chemistry and in dosimeters.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seekmedical attention immediately. If this chemical contactsthe skin, remove contaminated clothing and wash immediately with soap and water. Seek medical attention immediately. If this chemical has been inhaled, remove fromexposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing hasstopped and CPR if heart action has stopped. Transferpromptly to a medical facility. When this chemical hasbeen swallowed, get medical attention. Give large quantities of water and induce vomiting. Do not make an unconscious person vomit.Note to physician: For severe poisoning do not use BAL[British Anti-Lewisite, dimercaprol, dithiopropanol(C3H8OS2)] as it is contraindicated or ineffective in poisoning from iron.
storage
Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Prior to working with thischemical you should be trained on its proper handling andstorage. Store in tightly closed containers in a cool, wellventilated area away from light.
Shipping
.
Purification Methods
A solution in warm water (0.67g/mL) is cooled rapidly to 0o, and the resulting light bluish-green monoclinic crystals are filtered at the pump, washed with cold distilled water and pressed between sheets of filter paper to dry it. The solubility at 25o is 0.36g/mL. It separates as an almost white powder when a saturated aqueous solution is diluted with EtOH.
Properties of Ammonium iron(II) sulfate
| Melting point: | 100 °C |
| Density | 1.865 |
| solubility | soluble in H2O; insoluble in ethanol |
| form | Liquid |
| color | pale green |
| Water Solubility | g/100g H2O: 12.5 (0°C), 17.2 (10°C), 26.4 (20°C), 33 (30°C), 46 (40°C); insoluble alcohol [MER06] [LAN05] |
| Merck | 13,523 |
| CAS DataBase Reference | 10045-89-3(CAS DataBase Reference) |
| EPA Substance Registry System | Ferrous ammonium sulfate (10045-89-3) |
Safety information for Ammonium iron(II) sulfate
Computed Descriptors for Ammonium iron(II) sulfate
Ammonium iron(II) sulfate manufacturer
Techno Pharmchem
Related products of tetrahydrofuran
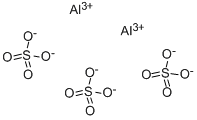
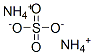
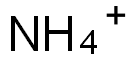
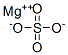
You may like
-
 10045-89-3 Ferrous Ammonium Sulphate 99%View Details
10045-89-3 Ferrous Ammonium Sulphate 99%View Details
10045-89-3 -
 10045-89-3 98%View Details
10045-89-3 98%View Details
10045-89-3 -
 Ammonium iron(II) sulfate 98%View Details
Ammonium iron(II) sulfate 98%View Details
10045-89-3 -
 37951-47-6 3'-Benzyloxy propiophenone, 98% 99%View Details
37951-47-6 3'-Benzyloxy propiophenone, 98% 99%View Details
37951-47-6 -
 104944-18-5 99%View Details
104944-18-5 99%View Details
104944-18-5 -
 3'-Methoxypropiophenone, 99% 37951-49-8 99%View Details
3'-Methoxypropiophenone, 99% 37951-49-8 99%View Details
37951-49-8 -
 694-48-4 99%View Details
694-48-4 99%View Details
694-48-4 -
 51364-51-3 99%View Details
51364-51-3 99%View Details
51364-51-3