1336-21-6
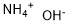
Product Name:
Ammonium hydroxide
Formula:
H5NO
Synonyms:
Ammonia aqueous;Ammonia Solution;Ammonia water;Ammonium hydroxide solution;Ammonium hydroxide solution, Ammonia water
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Ammonium hydroxide appears as a colorless aqueous solution. Concentration of ammonia ranges up to approximately 30%. Ammonia vapors (which arise from the solution) irritate the eyes. |
|---|---|
| Color/Form | Colorless liquid |
| Odor | Intense, pungent, suffocating odor |
| Taste | Acrid taste |
| Boiling Point | 38 °C (25%) |
| Melting Point | -58 °C (25%) |
| Solubility | Exists only in solution |
| Density | About 0.90 @ 25 °C/25 °C |
| Vapor Density | Relative vapor density (air = 1): 0.6 |
| Vapor Pressure | 2160 mm Hg @ 25 °C |
| Decomposition | When heated to decomposition it emits ammonia and nitroxides. |
| Corrosivity | Dissolves copper, zinc |
| pH | pH= 11.6 (1.0 N solution); 11.1 (0.1 N solution); 10.6 (0.01 N solution) |
| Odor Threshold | Odor Threshold Low: 50.0 [ppm] [HSDB] Odor threshold from CHRIS |
| Dissociation Constants | pKb= 4.767, Kb= 1.710X10-5 at 20 °C; pKb= 4.751, Kb= 1.774X10-5 at 25 °C; pKb= 4.740, Kb= 1.820X10-5 at 30 °C |
| Other Experimental Properties | Reaction with sulfuric acid or other strong mineral acids is exothermic; mixture becomes boiling hot |
| Chemical Classes | Other Classes -> Bases |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 35.046 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 0 |
| Exact Mass | 35.037113783 g/mol |
| Monoisotopic Mass | 35.037113783 g/mol |
| Topological Polar Surface Area | 2 Ų |
| Heavy Atom Count | 2 |
| Formal Charge | 0 |
| Complexity | 0 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 2 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Ammonium hydroxide appears as a colorless aqueous solution. Concentration of ammonia ranges up to approximately 30%. Ammonia vapors (which arise from the solution) irritate the eyes.
RELATED SUPPLIERS
Cynor Laboratories
Gujarat
product:AMMONIA SOLUTION 25% AR (1LTR), 1336-21-6, Grade Standard: Reagent Grade
