Amineptine
- CAS NO.:57574-09-1
- Empirical Formula: C22H27NO2
- Molecular Weight: 337.46
- EINECS: 260-818-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-15 10:44:02
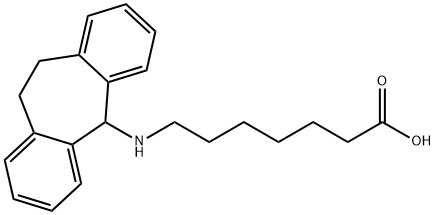
What is Amineptine?
Originator
Survector,Eutherapie,France,1978
The Uses of Amineptine
Amineptine is used as an atypical tricyclic antidepressant. Amineptine selectively inhibits the reuptake of dopamine and to a lesser extent norepinephrine.
Definition
ChEBI: A carbocyclic fatty acid that is 5-aminoheptanoic acid in which one of the hydrogens attached to the nitrogen is replaced by a 10,11-dihydro-5H-dibenzo[a,d][7]annulen-5-yl group. A tricyclic antidepressant, it w s never approved in the US and was withdrawn from the French market in 1999 due to concerns over abuse, dependence and severe acne.
Manufacturing Process
6.5 g of 5-chloro-10,11-dihydro-5H-dibenzo[a,d]cycloheptene in 60 ml of
nitromethane and 10.8 g of ethyl 7-aminoheptanoate in 12ml of nitromethane
were mixed at ambient temperature. The reaction was slightly exothermic.
The reaction mixture was left to stand overnight and the solvent was
evaporated in vacuum. The residue was taken up in normal hydrochloric acid
and the resulting precipitate was filtered off.
10.5 g of crude ethyl 7-[dibenzo(a,d)cycloheptadiene-5-yl]aminoheptanoate
hydrochloride were obtained, of which a sample recrystallized from benzene
gave a pure product melting instantaneously at 166°C to 168°C.
The hydrochloride of the crude ester obtained above was added to 25 ml of 2
N hydrochloric acid. The whole was kept under reflux for 2 hours. The
material dissolved and a new hydrochloride then reprecipitated. After cooling,
the hydrochloride of the crude acid was filtered off, washed with iced water
and then recrystallized from distilled water. 5.7 g of 7-
[dibenzo(a,d)cycloheptadienb-yl] aminoheptanoic acid hydrochloride were
obtained, melting instantaneously at 226°C to 230°C.
Therapeutic Function
Central stimulant
Enzyme inhibitor
This synthetic dopamine reuptake inhibitor (FW = 338.47 g/mol; CAS 57574-09-1), also known by the trade names Survector?, Maneon?, Directim?, Neolior?, Provector?, and Viaspera? as well as its IUPAC name, 7-[(10,11-dihydro-5H-dibenzo[a,d]-cyclohepten-5-yl)amino]heptanoic acid, significantly reduces the effect of 6-hydroxy-dopamine on brain dopamine, increases striatal homovanillic acid concentrations, and shows cross-tolerance. Despite these amphetamine-like properties, amineptine does not alter brain noradrenaline or acetylcholine concentrations, suggesting amineptine is a new type of antidepressant with a brain biochemical profile differing from that of other drugs used in depressive disorders. Amineptine is also a norepinephrine reuptake inhibitor. This agent is also ineffective in reducing amphetamine withdrawal symptoms or craving. Amineptine failed to secure FDA approval and is not a duly authorized drug in the U.S.
Properties of Amineptine
| Melting point: | 209 °C |
| Boiling point: | 509.9±50.0 °C(Predicted) |
| Density | 1.13±0.1 g/cm3(Predicted) |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| pka | 4.75±0.10(Predicted) |
| form | Solid |
| color | White to Pale Yellow |
| NIST Chemistry Reference | Amineptine(57574-09-1) |
Safety information for Amineptine
Computed Descriptors for Amineptine
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1