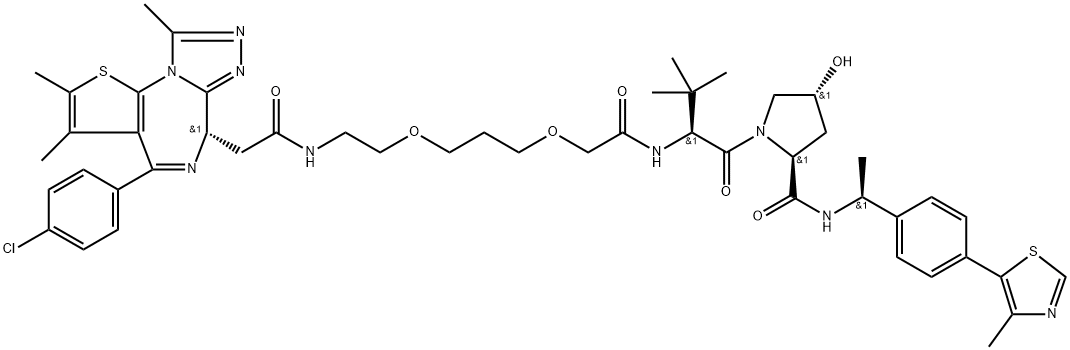
AMG 510
- CAS NO.:2252403-56-6
- Empirical Formula: C30H30F2N6O3
- Molecular Weight: 560.59
- Update Date: 2024-11-19 15:53:33
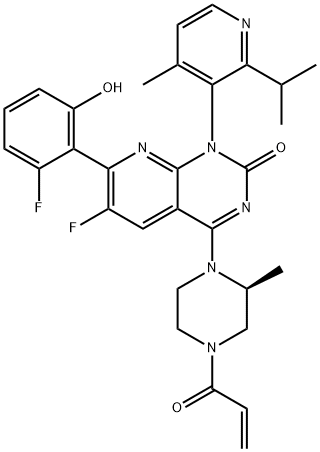
What is AMG 510?
Absorption
A 960 mg once daily dose of sotorasib reaches a Cmax of 7.50 μg/mL, with a median Tmax of 2.0 hours, and an AUC0-24h of 65.3 h*μg/mL.
Toxicity
Data regarding overdoses of sotorasib are not readily available. However, in clinical trials, signs of dose limiting toxicity were not found. Patients experiencing an overdose may experience and increased risk and severity of adverse effects such as diarrhea, nausea, vomiting, fatigue, and elevated aminotransferase.
The Uses of AMG 510
AMG-510 is a KRAS G12C inhibitor thus leading to the regression of KRAS G12C tumors as it interrupts protein signaling.
Indications
Sotorasib is indicated in the treatment of KRAS G12C-mutated locally advanced or metastatic non-small cell lung cancer (NSCLC) in adults who have received at least one prior systemic therapy.
Background
Sotorasib, also known as AMG-510, is an acrylamide-derived KRAS inhibitor developed by Amgen. It is indicated in the treatment of adult patients with KRAS G12C mutant non-small cell lung cancer. This mutation makes up >50% of all KRAS mutations. Mutant KRAS discovered in 1982 but was not considered a druggable target until the mid-2010s. It is the first experimental KRAS inhibitor. The drug MRTX849 is also currently being developed and has the same target.
Sotorasib was granted FDA approval on May 28, 2021, followed by the European Commission's approval on January 10, 2022.
Definition
ChEBI: Sotorasib is a pyridopyrimidine that is pyrido[2,3-d]pyrimidin-2(1H)-one substituted by 4-methyl-2-(propan-2-yl)pyridin-3-yl, (2S)-2-methyl-4-(prop-2-enoyl)piperazin-1-yl, fluoro and 2-fluoro-6-hydroxyphenyl groups at positions 1, 4, 6 and 7, respectively. It is approved for the treatment of patients with non-small cell lung cancer having KRAS(G12C) mutations. It has a role as an antineoplastic agent. It is a member of acrylamides, a N-acylpiperazine, a pyridopyrimidine, a member of monofluorobenzenes, a member of methylpyridines, a tertiary carboxamide, a tertiary amino compound and a member of phenols.
Pharmacokinetics
Sotorasib is indicated in the treatment of adults with KRAS G12C mutant non small cell lung cancer. It has a moderate duration of action as it is given daily. Patients should be counselled regarding the risks of hepatotoxicity, interstitial lung disease and pneumonitis; and to avoid breastfeeding during treatment and up to 1 week after the last dose.
in vitro
It was examined whether AMG510 has an antitumor effect on the CACO-2 (human colorectal cancer) cells into which the KRAS G12C (Kirsten rat sarcoma 2 viral oncogene homolog mutation) gene was introduced. The results showed that there was a clear concentration dependent RPR (relative proliferation ratio) decrease and that AMG 510 selectively inhibited KRAS G12C in colon cancer cells in vitro (Fig. 4A). Reference: Mol Clin Oncol. 2021 Jul;15(1):148.
Metabolism
Sotorasib is predominantly metabolized through conjugation or by CYP3As.
storage
Store at -20°C
Properties of AMG 510
| Boiling point: | 730.5±70.0 °C(Predicted) |
| Density | 1.36±0.1 g/cm3(Predicted) |
| storage temp. | Store at -20°C, stored under nitrogen |
| solubility | DMSO:75.0(Max Conc. mg/mL);133.78(Max Conc. mM) Water:33.33(Max Conc. mg/mL);59.45(Max Conc. mM) Ethanol:8.0(Max Conc. mg/mL);14.27(Max Conc. mM) |
| form | Solid |
| pka | 6.52±0.35(Predicted) |
| color | Light yellow to yellow |
Safety information for AMG 510
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for AMG 510
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-(2,4-Dimethoxybenzyl)dihydropyrimidine-2,4(1H,3H)-dione 7-Bromo-1H-indazole N-octanoyl benzotriazole 3,4-Dibenzyloxybenzaldehyde 4-Hydrazinobenzoic acid Electrolytic Iron Powder Fmoc-Val-Cit-PAB 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 4-HYDROXY BENZYL ALCOHOL 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) S-2-CHLORO PROPIONIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 1-(4-Methylphenylsulfonyl)-1H-1,2,3-benzotriazole 1-(2-Chlorobenzyl)-4-nitro-1H-pyrazole 1-(2-Nitrophenyl)-4-phenylpiperazineRelated products of tetrahydrofuran
You may like
-
 55441-95-7 2 2-BIS(2-HYDROXYETHOXY)-1 1-BINAPHTHYL 99%View Details
55441-95-7 2 2-BIS(2-HYDROXYETHOXY)-1 1-BINAPHTHYL 99%View Details
55441-95-7 -
 181228-33-1 99%View Details
181228-33-1 99%View Details
181228-33-1 -
 Ste-Glu-AEEA-AEEA-OSUView Details
Ste-Glu-AEEA-AEEA-OSUView Details
1169630-40-3 -
 1446013-08-6 Fmoc-His-Aib-OH TFA 98%View Details
1446013-08-6 Fmoc-His-Aib-OH TFA 98%View Details
1446013-08-6 -
 127464-43-1 99%View Details
127464-43-1 99%View Details
127464-43-1 -
 Chloro Uracil 99%View Details
Chloro Uracil 99%View Details
1820-81-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0 -
 13162-05-5 99%View Details
13162-05-5 99%View Details
13162-05-5