Auranofin
Synonym(s):1-Thio-β-D-glucopyranosatotriethylphosphine gold-2,3,4,6-tetraacetate;3,4,5-Triacetyloxy-6-(acetyloxymethyl) oxane-2-thiolate triethylphosphanium;SKF 39162
- CAS NO.:34031-32-8
- Empirical Formula: C20H34AuO9PS
- Molecular Weight: 678.48
- MDL number: MFCD00080759
- EINECS: 251-801-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
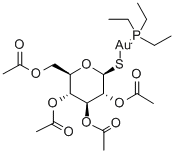
What is Auranofin?
Toxicity
Oral, rat: LD50 = > 2000 mg/kg. Symptoms of overdose may include diarrhoea, vomiting, abdominal cramps, and symptoms of hypersensitivity (such as skin rash, hives, itching, and difficulty breathing).
Description
Auranofin is the first orally effective gold compound to be marketed for the treatment of severe rheumatoid arthritis. It is better tolerated and more convenient than gold sodium thiomalate, which is administered intramuscularly.
Chemical properties
White to Off-White Solid
Originator
Smith Kleiu & French (USA)
The Uses of Auranofin
Auranofin is a new oral gold-based antiarthritis drug. Auranofin inhibits various leukocyte activation pathways at multiple sites. Auranofin inhibits the release of inflammatory mediators from human m acrophages, basophils, and pulmonary mast cells. Auranofin is an efficient inducer of mitochondrial membrane permeability transition pore in the presence of calcium ions related to its inhibition of m itochondrial thioredoxin reductase.
The Uses of Auranofin
Auranofin inhibits both leukocyte activation pathways at multiple sites and the release of inflammatory mediators from human macrophages, basophils, and pulmonary mast cells. Auranofin also inhibits 5-lipoxygenase in human neutrophils, IKB kinase (IKK) by modifying Cys-179 of the IKKβ subunit 5 and selenoenzyme thioredoxin reductase (TrxR) which is involved in the defense against oxidative stress. Auranofin is a disease-modifying antirheumatic drug (DMARD) and has been used to study the anti-proliferative effects against OVCAR-3 human ovarian carcinoma cells.
Background
Auranofin is a gold salt that is capable of eliciting pharmacologic actions that suppress inflammation and stimulate cell-mediated immunity. It has subsequently been listed by the World Health Organization as a member of the antirheumatic agent category. Auranofin appears to induce heme oxygenase 1 (HO-1) mRNA. Heme oxygenase 1 is an inducible heme-degrading enzyme with anti-inflammatory properties.
What are the applications of Application
Auranofin is a gold(I)-phosphine thiolate small molecule described to produce antiinflammatory and antiarthritic effects and an inhibitor of 5-LO (5-lipoxygenase)/LTA synthase
Indications
Used in the treatment of active, progressive or destructive forms of inflammatory arthritis, such as adult rheumatoid arthritis.
Definition
ChEBI: An S-glycosyl compound consisting of 2,3,4,6-tetra-O-acetyl-1-thio-beta-D-glucopyranose with the sufur atom coordinated to (triethylphosphoranylidene)gold. It is administered orally fo the treatment of active progressive rheumatoid arthritis.
Manufacturing Process
(A) Triethylphosphine gold chloride: A solution of 10.0 g (0.08 mol) of
thiodiglycol in 25 ml of ethanol is mixed with a solution of 15.76 g (0.04 mol)
of gold acid chloride trihydrate in 75 ml of distilled water. When the bright
orange-yellow solution is almost colorless, it is cooled to -5°C and an equally
cold solution of 5.0 g (0.0425 mol) of triethylphosphine in 25 ml of ethanol is
added dropwise to the stirred solution. After the addition is complete, the
cooled mixture is stirred for ? hour. Solid that separates is removed and the
filtrate is concentrated to about 30 ml to yield a second crop. The combined
solid is washed with aqueous-ethanol (2:1) and recrystallized from ethanol by
adding water to the cloud point. The product is obtained as white needles, MP
85° to 86°C.
(B) Auranofin: A cold solution of 1.66 g (0.012 mol) of potassium carbonate in 20 ml of distilled water is added to a solution of 5.3 g (0.011 mol) of S-
(2,3,4,6-tetra-O-acetylglucopyranosyl)-thiopseudourea hydrobromide
[Methods in Carbohydrate Chemistry, vol 2, page 435 (1963)] in 30 ml of
water at -10°C. A cold solution of 3.86 g (0.011 mol) of triethylphosphine
gold chloride in 30 ml of ethanol containing a few drops of methylene chloride
is added to the above mixture before hydrolysis of the thiouronium salt is
complete. After the addition is complete, the mixture is stirred in the cold for
? hour. The solid that separates is removed, washed first with aqueous
ethanol then water and dried in vacuum. There is obtained colorless crystals,
MP 110° to 111°C, of S-triethylphosphine gold 2,3,4,6-tetra-O-acetyl-1-thio-β-
D-glucopyranoside.
brand name
Ridaura (Promethus, Smith Kline Beecham, USA), Aktil (Lek, Yugoslavia), Ridauran (Robapharm, France).
Therapeutic Function
Antiarthritic
Pharmaceutical Applications
Auranofin ([tetra-O-acetyl-β-D- (glucopyranosyl)thio]-triethylphosphine)gold(I) is a second-generation gold-based drug, licensed as an orally available gold drug for the treatment of RA. It features a linear S Au P geometry, as shown by X-ray analysis. It is more lipophilic than the first-generation drugs, which makes oral administration possible. Treatment with Auranofin requires less visits to the clinic, but it is believed to be less successful in the treatment of RA compared to gold drugs being administered intramuscularly.
Biochem/physiol Actions
Auranofin inhibits various leukocyte activation pathways at multiple sites. Auranofin inhibits the release of inflammatory mediators from human macrophages, basophils, and pulmonary mast cells. The compound also inhibits 5-lipoxygenase in human neutrophils. Auranofin is a disease-modifying antirheumatic drug (DMARD). The compound is a potent inhibitor of selenoenzyme thioredoxin reductase (TrxR), which is involved in defense against oxidative stress. Auranofin is an efficient inducer of mitochondrial membrane permeability transition pore in the presence of calcium ions related to its inhibition of mitochondrial thioredoxin reductase. Auranofin inhibits IKB kinase (IKK) by modifying Cys-179 of the IKKβ subunit 5.
Pharmacokinetics
Auranofin is a gold salt used in treating inflammatory arthritis. Gold salts are called second-line drugs because they are often considered when the arthritis progresses in spite of antiinflammatory drugs (NSAIDs and corticosteroids).
Clinical Use
Auranofin is indicated in adults with active rheumatoid arthritis who have not responded sufficiently to one or more NSAIDs.
Safety Profile
Poison by ingestion,intraperitoneal, and intravenous routes. Human systemiceffects by ingestion: ulceration or bleeding from stomach.An experimental teratogen. Other experimentalreproductive effects. Human mutation data reported.When heated to decomp
Synthesis
Synthesis: ethanolic thiodiglycol is treated
first with aqueous gold(I) acid chloride trihydrate, then with ethanolic triethylphosphine to
give triethylphosphine gold(I) chloride, which is
added to an aqueous solution of S-(2,3,4,6-tetra-O-acetylglucopyranosyl)pseudothiourea hydrobromide and potassium carbonate to give the desired auranofin.
Veterinary Drugs and Treatments
Auranofin has been used to treat idiopathic polyarthritis and pemphigus foliaceous in dogs and cats. Several clinicians report that while auranofin may be less toxic, it also less efficacious than injectable gold (aurothioglucose).
Metabolism
Not Available
Metabolism
On a mg gold/kg basis, it is reported to be as effective in the rat adjuvant arthritis assay as the parenterally effective drugs. Daily oral doses produce a rapid increase in kidney and blood gold levels for the first 3 days of treatment, with a more gradual increase on subsequent administration. Plasma gold levels are lower than those attained with parenteral gold compounds. The major route of excretion is via the urine. Auranofin may produce fewer adverse reactions than parenteral gold compounds, but its therapeutic efficacy also may be less.
References
1) Columbo et al. (1990), Modulation of mediator release from human basophils and pulmonary mast cells and macrophages by auranofin; Biochem. Pharmacol., 39 285 2) Rigobello et al. (2002), Induction of mitochondrial permeability transition by auranofin, a gold(I)-phosphine derivative; Br. J. Pharmacol., 136 1162 3) Merck 14 878
Properties of Auranofin
| Melting point: | 112-115° |
| storage temp. | room temp |
| solubility | DMSO: ≥5mg/mL |
| form | solid |
| color | Crystals |
| Stability: | Stable for 2 years from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20° for up to 2 months. |
Safety information for Auranofin
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H361:Reproductive toxicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for Auranofin
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran

You may like
-
 Auranofin, for HPLC ≥98% CAS 34031-32-8View Details
Auranofin, for HPLC ≥98% CAS 34031-32-8View Details
34031-32-8 -
 Auranofin CAS 34031-32-8View Details
Auranofin CAS 34031-32-8View Details
34031-32-8 -
 17604-74-9 3-Pyridineacetonitrile, α-hydroxy- 98+View Details
17604-74-9 3-Pyridineacetonitrile, α-hydroxy- 98+View Details
17604-74-9 -
 131987-69-4 98+View Details
131987-69-4 98+View Details
131987-69-4 -
 Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
67967-07-1 -
 2-Propanamine, 1-chloro-, hydrochloride (9CI) 98+View Details
2-Propanamine, 1-chloro-, hydrochloride (9CI) 98+View Details
5968-21-8 -
 3-Iodophenylacetic acid 1878-69-9 98+View Details
3-Iodophenylacetic acid 1878-69-9 98+View Details
1878-69-9 -
 132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6
